Cell proliferation inhibitory proteins and polynucleotides, antisense polynucleotides to the polynucleotides, cell proliferation inhibitors using the foregoing, cancer diagnostic agents, cancer therapeutic agents and compositions for gene therapy
a cell proliferation inhibitor and polynucleotide technology, applied in the field of gene information, can solve the problems of human cell aging, immortalization, or cancerization of human cells, and the correlation between these genes and aging,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning Immortalization-Related Genes
1. Culturing the First Generation Fibroblast Line
[0093] Human fibroblasts were prepared from a female embryo of 9 weeks old to form the first generation fibroblast line (KMS-6) (Namba, M. et al., Int. J. Cancer, 35: 275-280, 1985). The cells were cultured with an Eagle minimum medium (MEM available from GIBCO) containing 10% fetal bovine serum (FBS available from Sanko Pure Chemicals Co. Ltd.) or with a Dalbeco modified Eagle medium (DMEM available from GIBCO) under the conditions of 5% CO2 concentration and 37° C. After the cells were grown until almost confluent state in a plate for 5 to 7 days, cells were diluted ¼-fold and subcultured.
2. Establishing an Immortal Cell Line by Treatment with 60Co Radiation
[0094] KMS-6 cells that had been cultured semiconfluent were radiated with 60Co γ-ray having a dose of from 200 to 400 rads 13 times and were subjected to radiation treatment with a total of 2,800 rads. Subsequently, subculturing was rep...
example 2
REIC Clone
1. Screening the E. coli Library
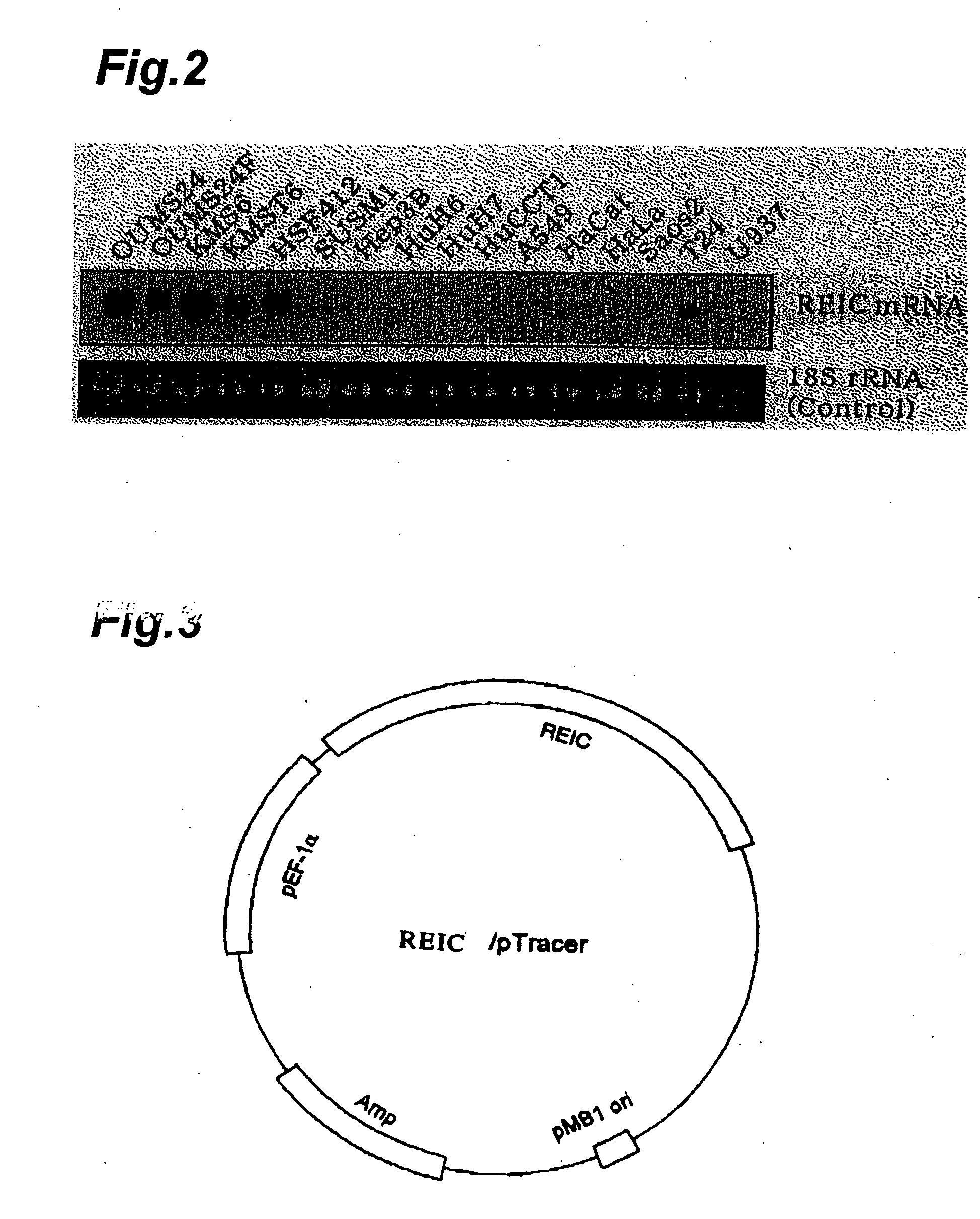
[0096] To remove pseudo-positive clones from the E. coli library, 32P labeled cDNA probe was prepared based on mRNA of OUMS-24F, a different immortal cell line (Bai, L. et al., Int. J. Cancer, 53: 451-456, 1993); the probe was used to carry out colony hybridization. The hybridization resulted in the removal of clones that had proved positive and the production of about 30 immortal candidate clones.
2. DNA Sequencing
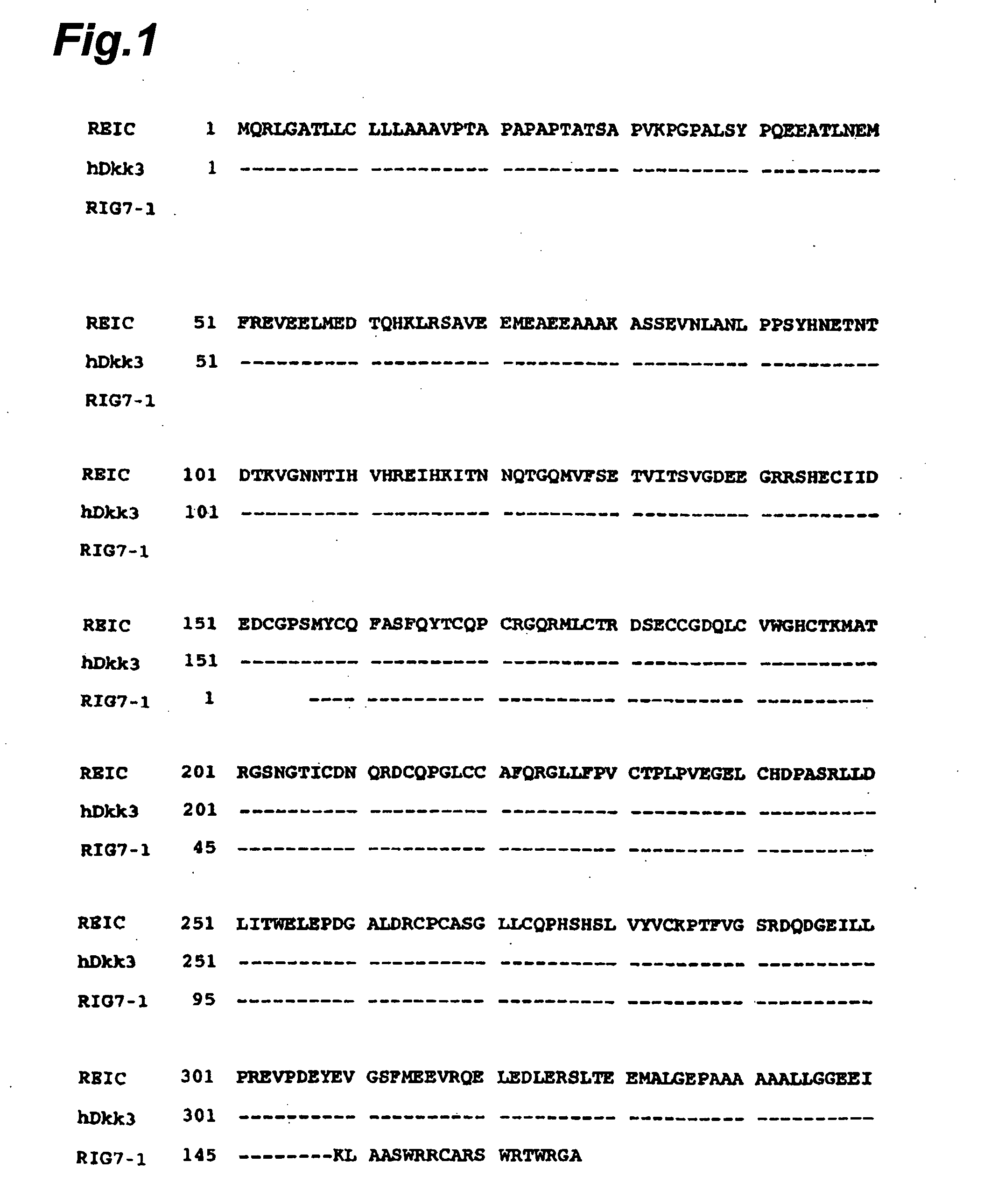
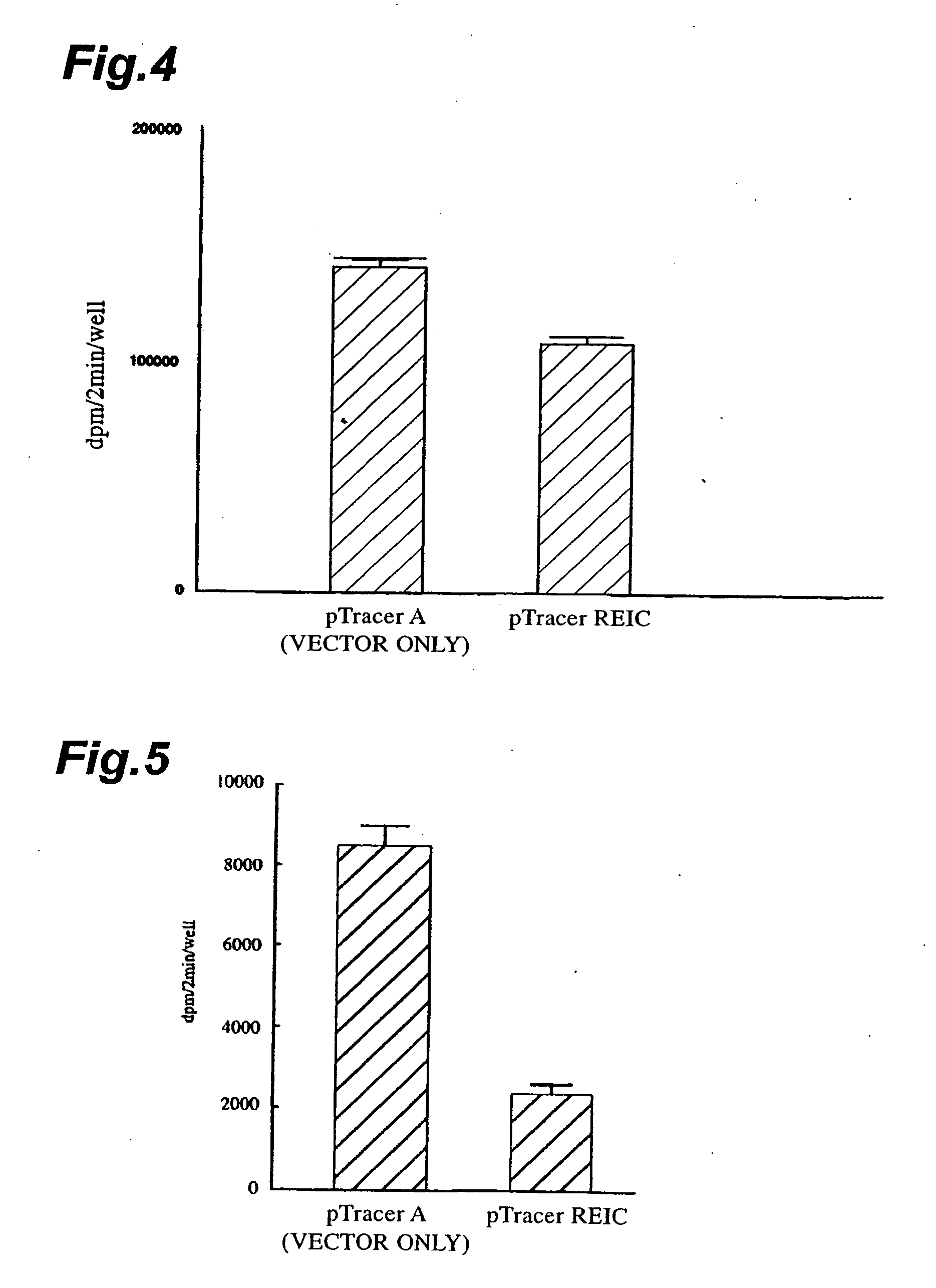
[0097] The DNA sequences of the clones obtained by screening were determined according to the Sanger method (Sanger F. et al., Proc. Natl. Acad. Sci. USA 74: 5463-5467, 1977). Consequently, clones were identified that had the aging-related gene sequences, including fibronectin, extracellular matrix proteins (e.g., α2 type I procollagen), collagenase, enzyme proteins (e.g., WS9-14), cell cycle regulating proteins (e.g., p21). Among these, it was found that REIC clone (D93) displaying no homology to known genes and being regard...
example 3
REIC Clone (10-1)
[0098] The DNA sequence of REIC clone (D93) predicted that the clone was not of the complete length cDNA. The polynucleotide having the DNA sequence set forth in SEQ ID NO:5 was used as a probe to screen a human heart cDNA library (BRL Inc.) for clones having cDNA fragments with longer DNA sequences. Consequently, there was obtained an REIC clone (10-1) that was predicted to carry the full-length cDNA containing the 5′-region of REIC (D93). DNA sequencing was conducted on this cDNA clone and the entire DNA sequence was determined. The DNA sequence of this clone is shown in SEQ ID NO:3 in the Sequence Listing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell proliferation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com