Delivery system for medical devices
a delivery system and medical device technology, applied in the field of medical device delivery systems, can solve the problems of inaccurate positioning of stents, prior art delivery systems, etc., and achieve the effects of improving the speed of delivering the catheter portion, facilitating minimal movement during device deployment, and accurate placemen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
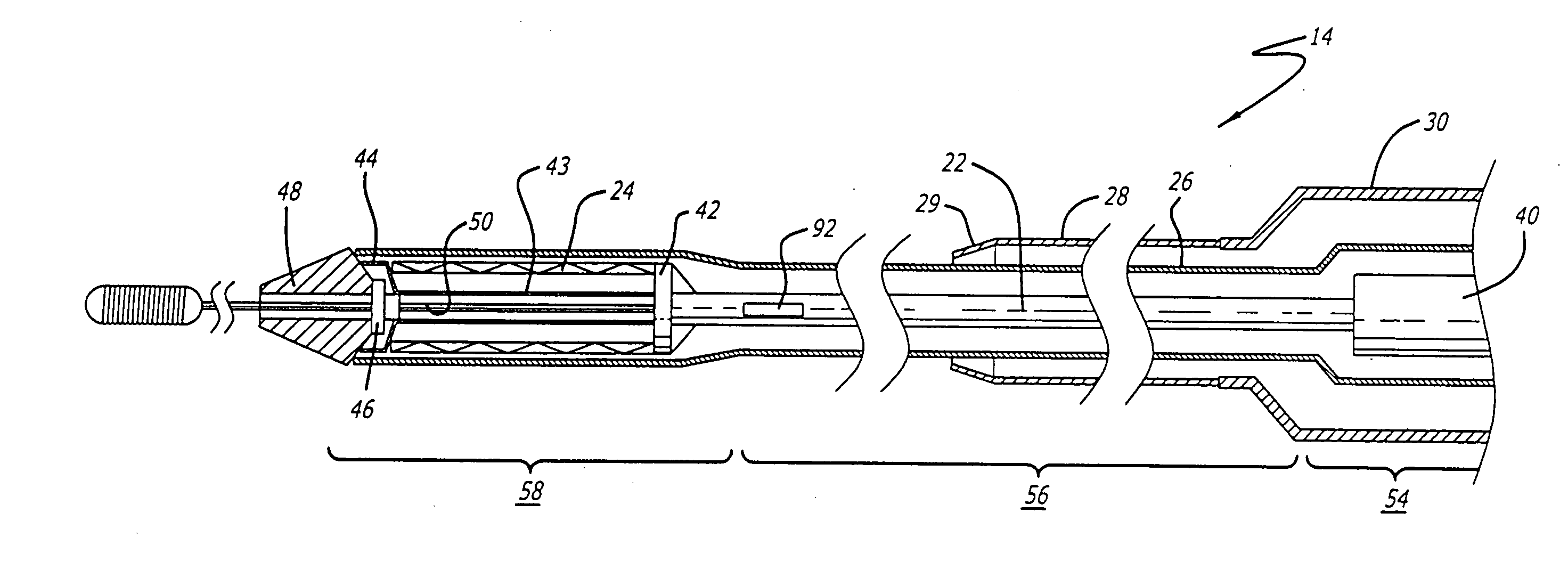
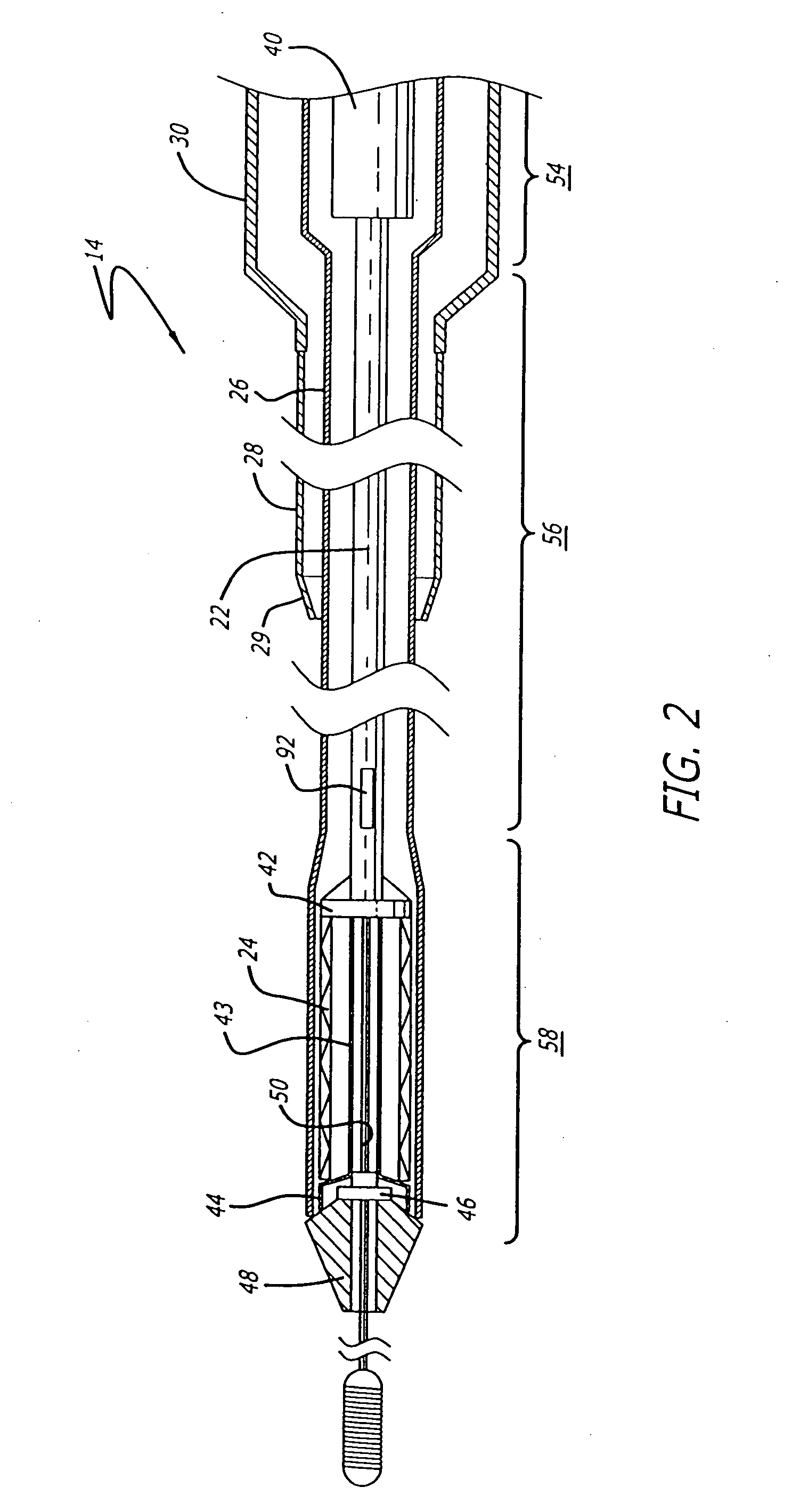
[0040] The present invention relates to a delivery system for delivering and deploying a medical device into a target site in a patient's body, such as a body lumen. For sake of illustration, the following exemplary embodiments are directed to a delivery system for delivering and deploying a self-expanding stent, although it is understood that the present invention is applicable to other medical devices which are implantable in a body lumen as well as other parts of the body. Additionally, the medical device can be either a self-expanding device or a non self-expanding device.
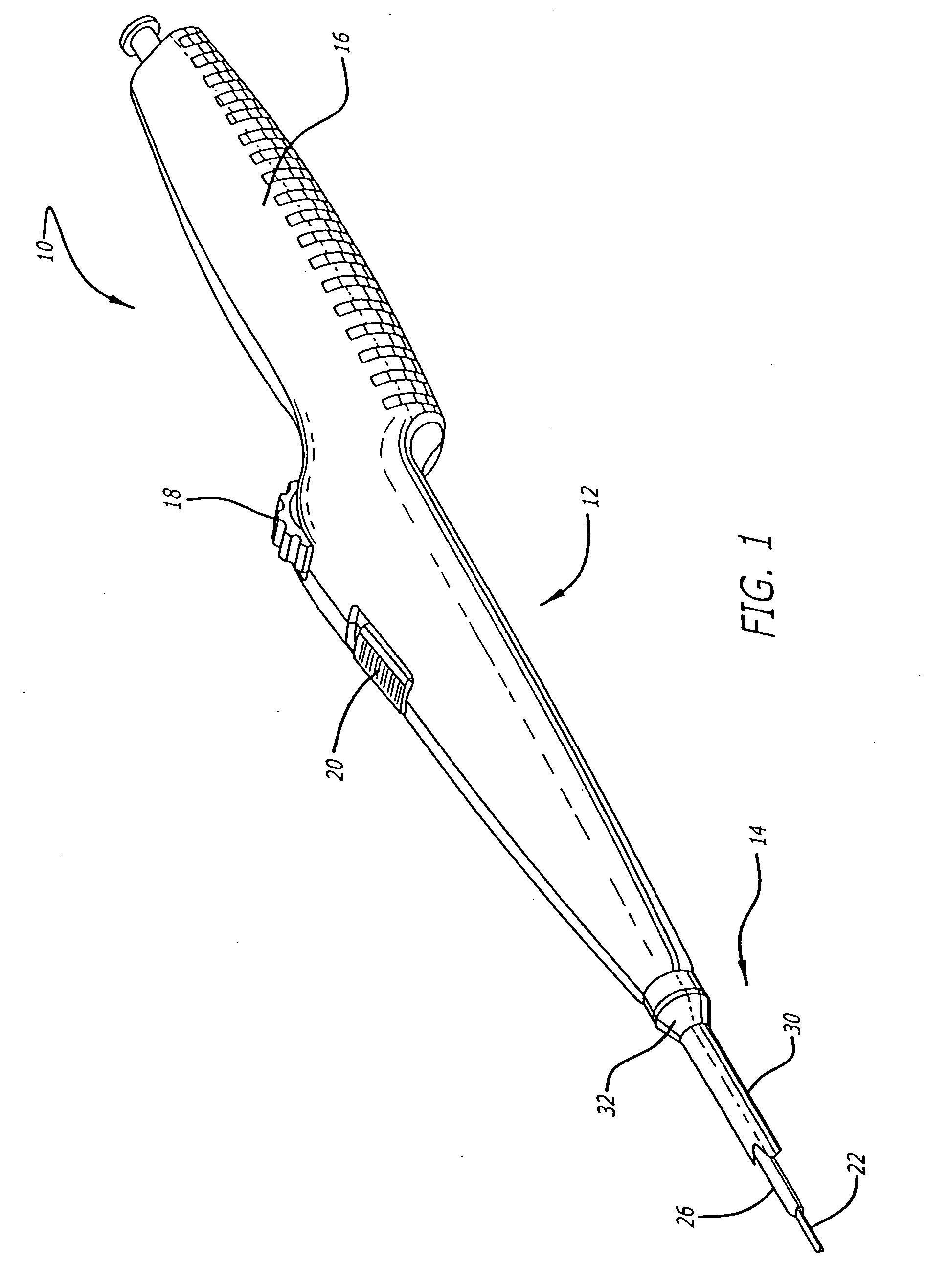
[0041] Referring now to FIGS. 1 and 2, in one particular embodiment of the present invention, the delivery system 10 incorporating features of the present invention includes a control handle 12 and a catheter portion 14. As can best be seen in FIG. 1, the control handle includes a hand portion 16 which allows the physician to hold the control handle utilizing one hand. The control handle 12 also includes a rot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com