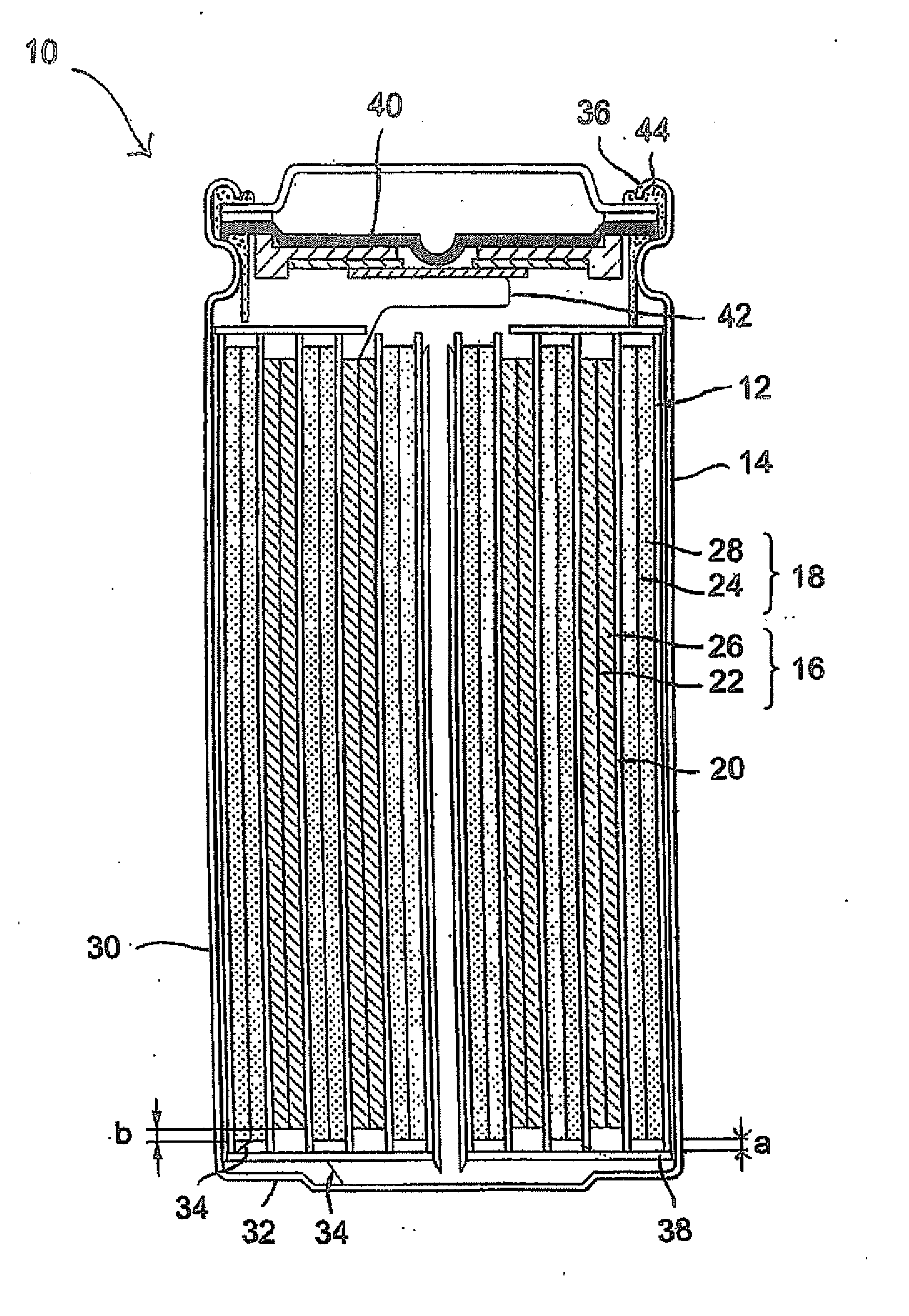
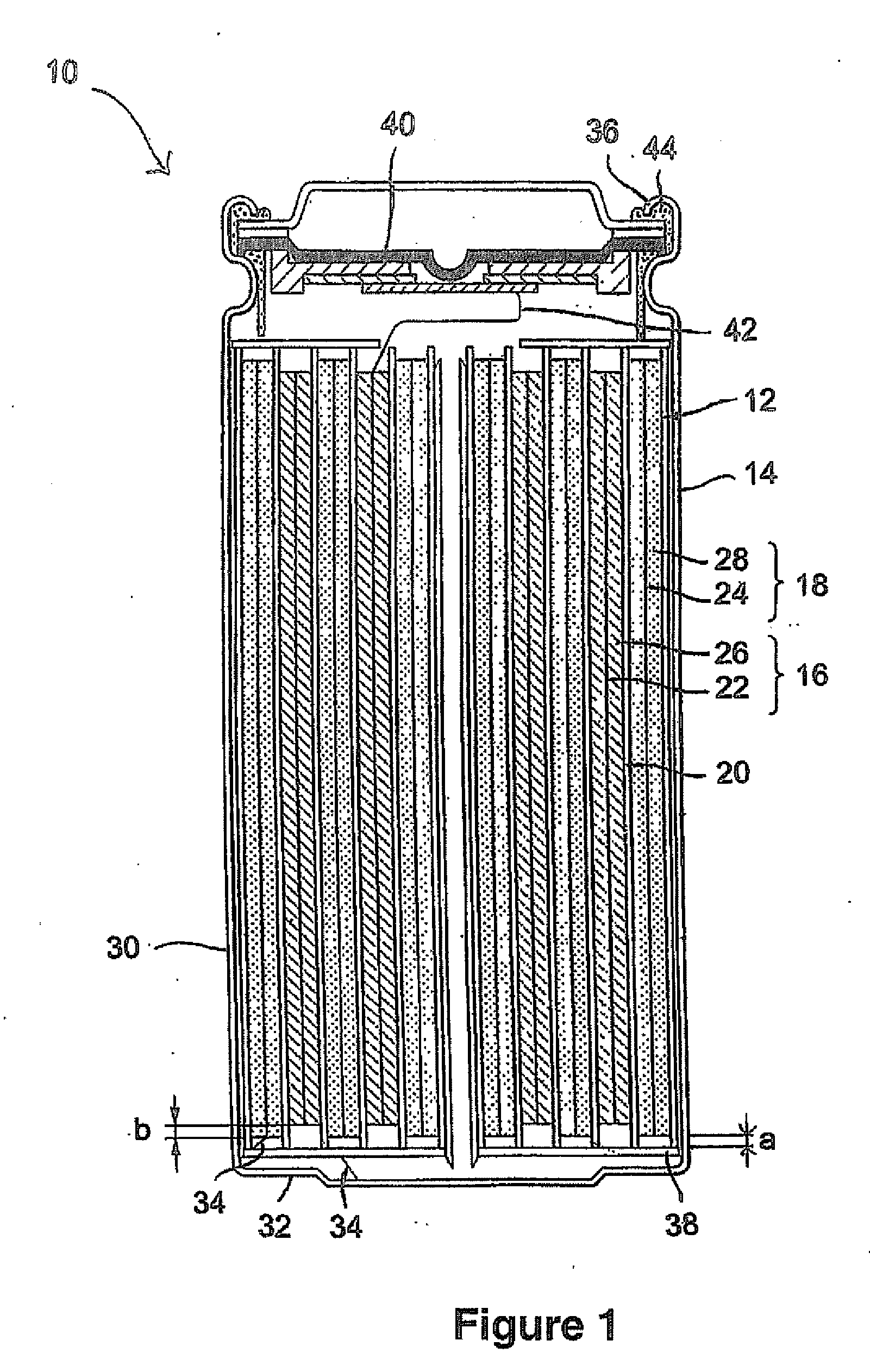
Method Of Making Active Materials For Use In Secondary Electrochemical Cells
a secondary electrochemical cell and active material technology, applied in the field of nanocrystalline vpo4 precursors, can solve the problem that materials are not always economical to produ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of VPO4
[0090] VPO4 was prepared according to the following reaction:
½ V2O5+(NH4)2HPO4+1.0 C→VPO4+2 NH3+3 / 2 H2O+CO
9.1 g V2O5, 13.2 g of (NH4)2HPO4 and 1.32 g of carbon (10% mass excess) were used. Carbon was added to the reaction mixture so that the V5+ in the V2O5 was reduced to V3+ in the product which is an example of carbothermal reduction. The excess carbon in the product helps act as a conducting agent in the vanadium phosphate electroactive materials produced therefrom, which improves the electrochemical properties of such electroactive materials.
[0091] It has been found that it is necessary to use a homogenous starting material. This can be achieved using high energy milling methods, which can include ball milling and micronizing. The sample prepared herein were prepared using McCrone micronizers to obtain the starting materials.
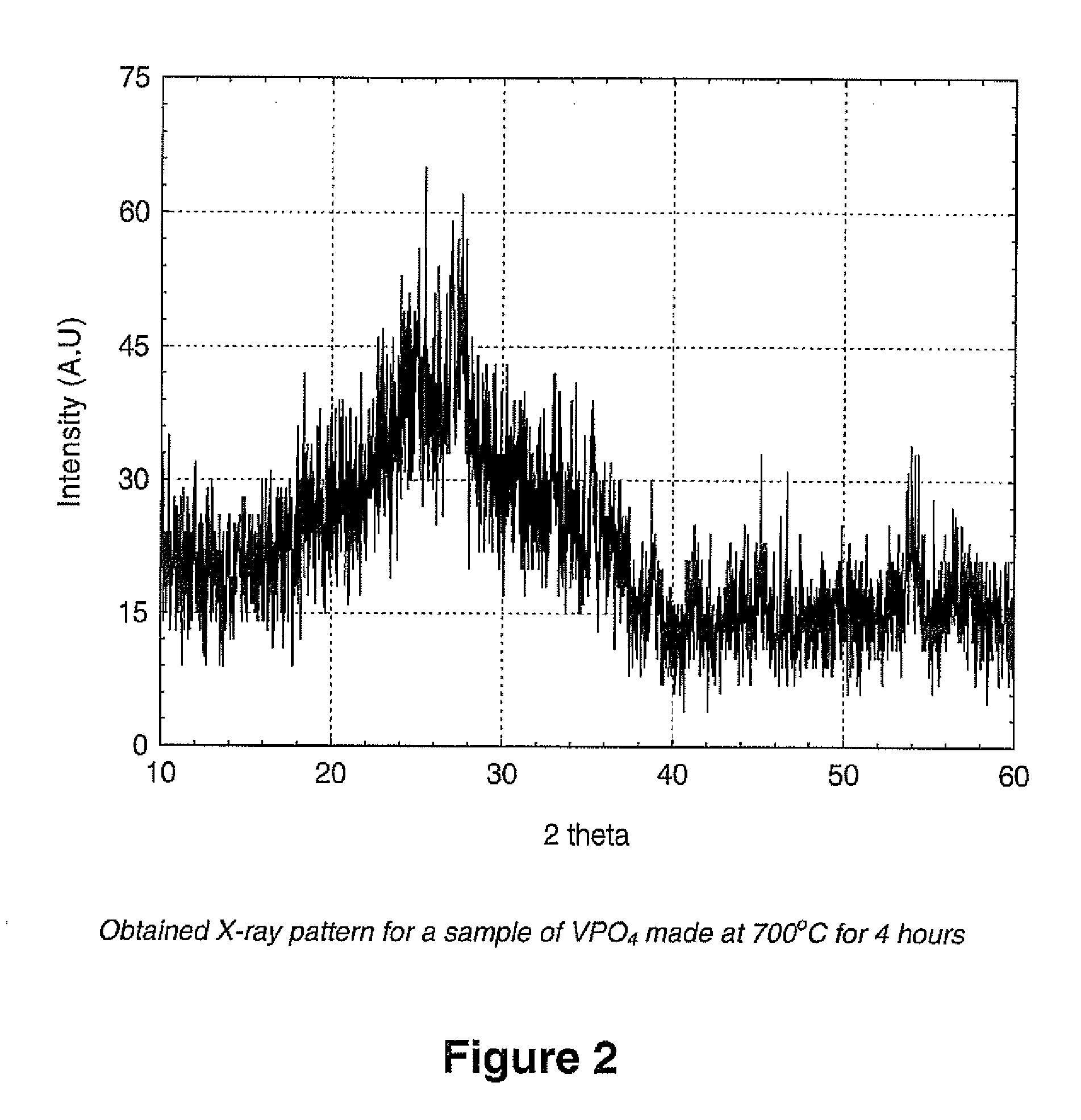
[0092] The VPO4 materials prepared herein were prepared at a number of different temperatures in the range from about 650° C. to abou...
example 2
Preparation of LiVPO4F using VPO4
[0094] LiVPO4 was prepared according to the following reaction:
LiF+VPO4→LiVPO4F
The LiF (2.6 g) and VPO4 (1.46 g) were mixed and micronized. The amount of LiF added is dependent on the amount of residual carbon present in the VPO4. The stoichiometric amount of LiF is added based on the above reaction. An allowance can be made for the amount of residual carbon left over from the V—P—O / C synthesis. This is normally about 3 weight percent. The mixture was then heated in the temperature range of about 600° to about 700° C. for up to about 1 hour. At temperatures in excess of 700° C., it is believed that VF3 sublimation occurs which leads to the formation of Li3V2(PO4)3 (LVP-nasicon).
[0095]FIG. 3 shows an example of a powder pattern obtained for a sample of LiVPO4F. Refinement of the XRD data for the LiVPO4F samples was carried out using the Rietveld method. H. M. Rietveld, J. Appl. Crystallograph, 2, (1969) 65. R. A. Young in “The Rietveld Method”, Cha...
example 3
[0100] An electrode active material comprising LiV1−xAlxPO4F was made according to the following reaction scheme:
(1−x)VPO4+x AlPO4+LiF →LiV1−xAlxPO4F
[0101] The LiF, VPO4 and AIPO4 were mixed and micronized in the required amounts. If for example x=0.2
0.8 VPO4+0.2 AlPO4+LiF→LiVo0.8Al0.2PO4F
Then 1.167 g of VPO4 were mixed with 0.244 g AlPO4 and 0.259 g LiF. The mixture was then heated in the temperature range 600-700° C. for up to 1 hour.
[0102] An electrode is made with 84% of the active material, 6% of Super P conductive carbon, and 10% poly vinylidene difluoride. A cell with that electrode as cathode and a lithium anode is constructed with an electrolyte comprising 1 M LiPF6 dissolved in 2:1 by weight mixture of ethylene carbonate:dimethyl carbonate.
[0103]FIG. 7 shows the X-ray powder patterns for samples of LiV1−xAlxPO4F. The results shown in FIG. 7 show that high quality samples can be prepared relatively easily.
[0104]FIG. 8 is a plot of Al content versus unit cell volume, obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com