Transdermal Therapeutic System
a transdermal therapeutic system and transdermal coating technology, applied in the field of transdermal therapeutic systems, can solve the problems of increasing the complexity of packaging, and increasing the cost of manufacturing and disposing of packaging, so as to reduce packaging costs, reduce packaging costs, and reduce packaging thickness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
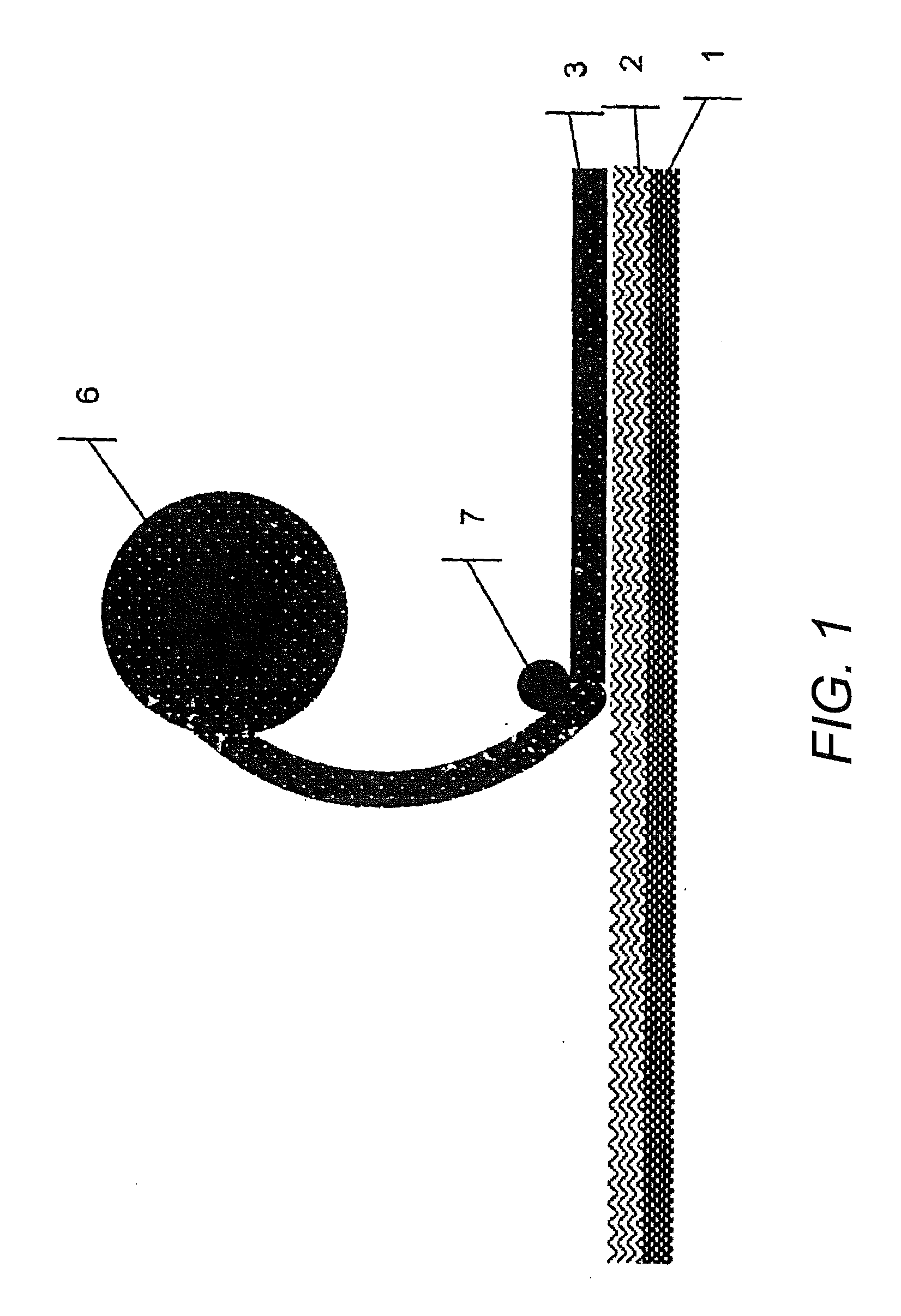
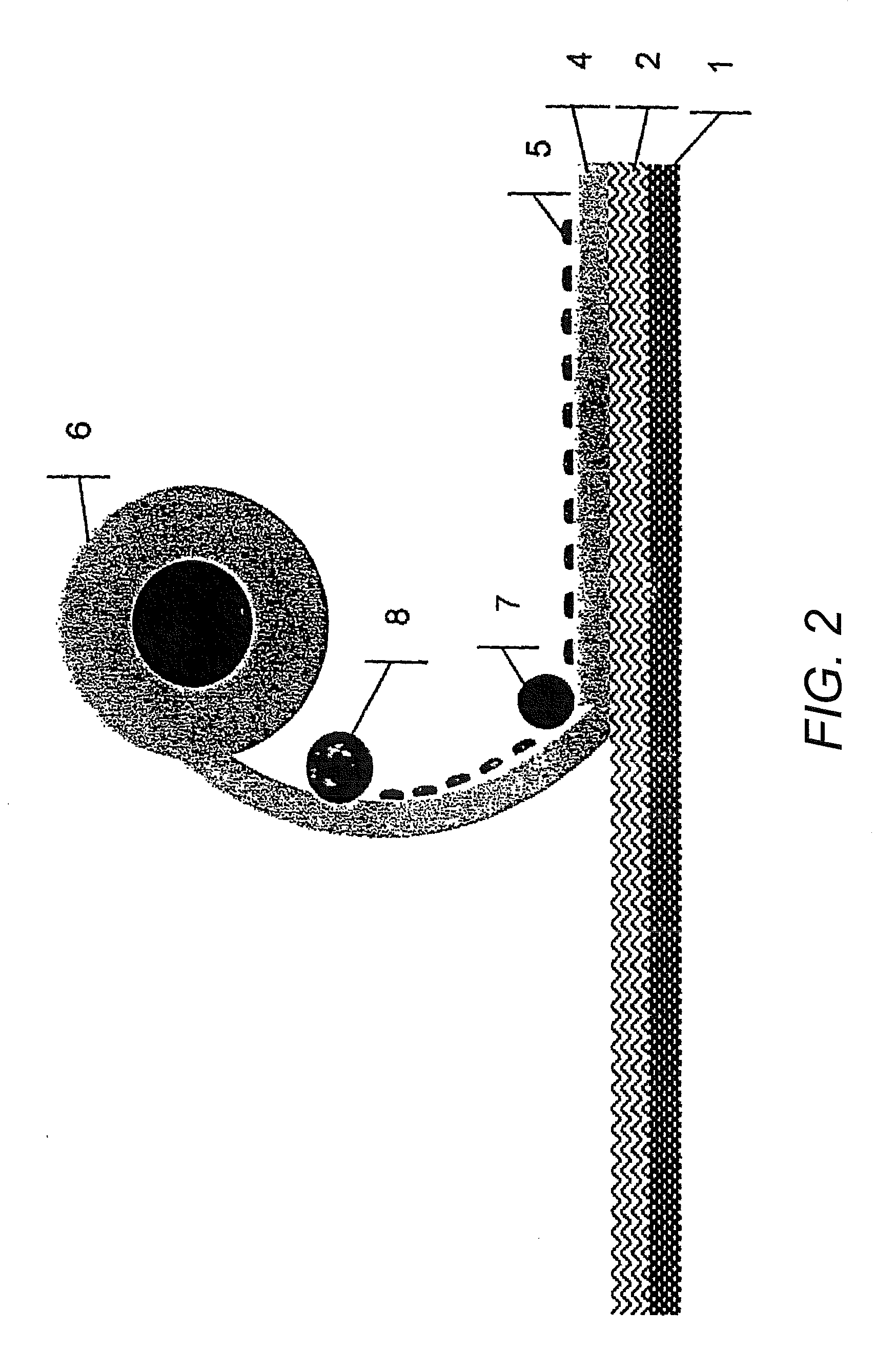
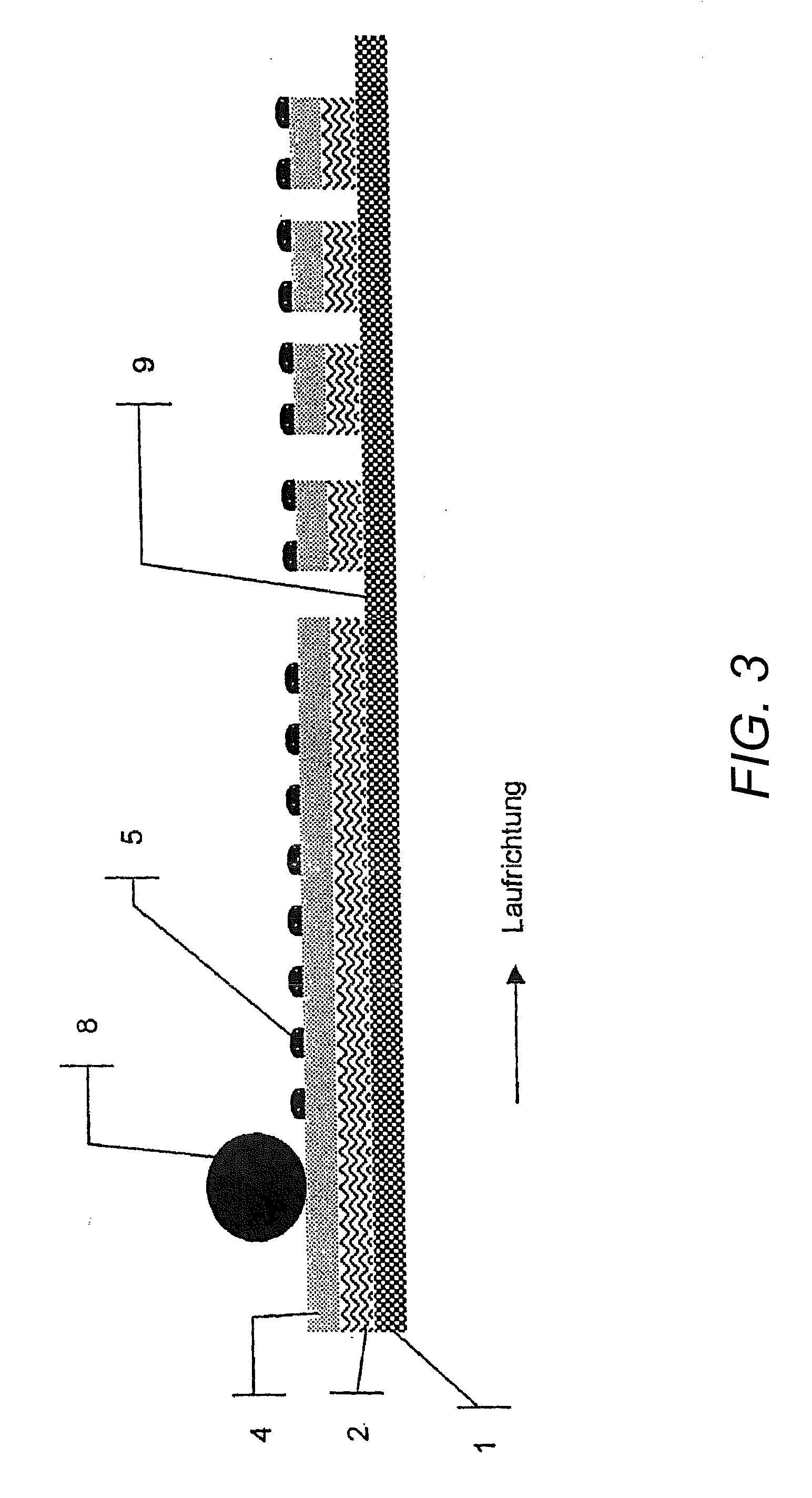
[0116] Manufacture of a cover layer that is impermeable to the active substances or removable protective layer of a transdermal therapeutic system, wherein such layers have been coated with a polymer support containing absorbing agents and channel-forming agents:
[0117] First, the components polymer, channel-forming agents, and absorbing agents are mixed at an elevated temperature, and preferably, the polymer and the channel-forming agents are premixed.
[0118] Portions of the resulting mixture are packaged for subsequent processing or processed directly. For that purpose, the mixture is applied in a molten state, e.g. in an extruder or by using other suitable mixing and / or dosing devices, the basic variations of which, for example, are already commonly used in the application of hot melt adhesives. Afterwards, the mixture is applied, for example by means of nozzles or rollers, onto the film which already forms or will form a part of the active substance-containing composite, in a de...
example 3
[0119] Composition of the Mixture:
Polypropylene70 percent by weightPolyethylene Glycol10 percent by weightMolecular Sieve 4 Å20 percent by weight
example 4
[0120] Composition of the Mixture:
Polypropylene35 percent by weightPolyethylene Glycol12 percent by weightMolecular Sieve 4 Å53 percent by weight(Baylith ® Powder)
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com