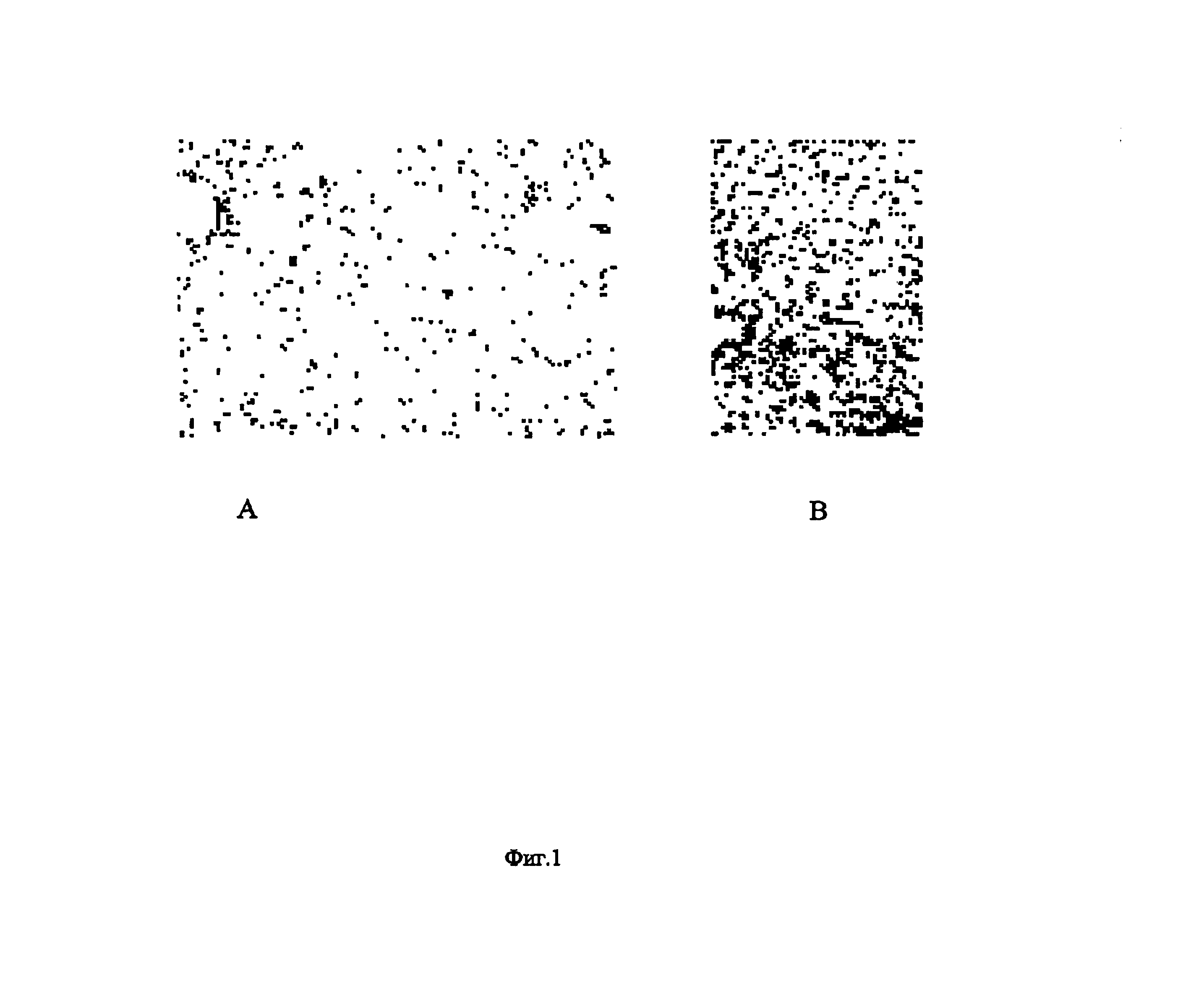
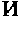Method for Treating Oncological, Virulent and Somatic Diseases, Method for Controlling Treatment Efficiency, Pharmaceutical Agents and Composition for Carrying Out Said Treatment
a technology for virulent and somatic diseases and oncology, applied in the field of medicine and veterinary science, can solve the problems of insufficient therapeutic effect and lack of data about the genetic repertoire of dna, and achieve the effect of increasing the efficacy of traditional therapeutic methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0083] Influence of DNase I Administrated Twice a Day on Erlich Tumor Growth at Mice
[0084] Group 1—10 mice transplanted with Erlich carcinoma (control).
[0085] Group 2—10 mice transplanted with Erlich carcinoma. Mice were intraperitoneally injected with DNase at 1 mg / kg dose in 200 ml of phosphate buffer twice a day starting from 3 up to 7 day after tumor transplantation.
[0086] Group 3—10 mice transplanted with Erlich carcinoma. Mice were intraperitoneally injected with DNase at 2 mg / kg dose in 200 ml of phosphate buffer twice a day starting from 3 up to 7 day after tumor transplantation.
[0087] Results of experiments were estimated according to tumor's growth inhibition (TGI) that were expressed in percents to control data received at last day of DNase injection with standard formula using.
[0088] Tumor's size on the 7 day after transplantation.
Tumor'sGroupvolumeTGI %P1 86+ / −12——233 + / − 661%p 334 + / − 760%p
[0089] The data obtained indicates that tumor's growth is significantly ...
example 2
Inhibition of Erlich Tumor Growth by Use of Different Schemes of DNase Administration
[0090] In our experiments DNA circulating in blood plasma of patients was the therapeutic target of DNase. Prolonged presence of DNase in blood plasma at catalytically effective concentrations is essential for provision of maximal therapeutic effect. According to that DNase myltiply administration should provide better therapeutic effect than the same total daily dose, administrated just as only two daily injections.
[0091] Group 1—10 mice transplanted with Erlich carcinoma (control).
[0092] Group 2—10 mice transplanted with Erlich carcinoma. Mice were intraperitoneally injected with DNase at 1 mg / kg dose in 200 ml of phosphate buffer twice a day starting from 3 up to 7 day after tumor transplantation.
[0093] Group 3—10 mice transplanted with Erlich carcinoma. Mice were intraperitoneally injected with DNase at 0.5 mg / kg dose in 200 ml of phosphate buffer four times a day starting from 3 up to 7 day...
example 3
Combined Use of DNase and Antitumor Doxorubicin Compound
[0097] Group 1—10 mice transplanted with Erlich carcinoma (control).
[0098] Group 2—10 mice replanted with Erlich carcinoma. Mice were intravenously injected with Doxorubicin at 2 mg / kg dose once a day starting from 3 up to 7 day after tumor transplantation.
[0099] Group 3—10 mice replanted with Erlich carcinoma. Mice were intravenously injected with Doxorubicin at 2 mg / kg dose once a day starting from 3 up to 7 day after tumor transplantation. As well mice were intraperitoneally injected with DNase at 0.5 mg / kg dose in 200 ml of phosphate buffer four times a day starting from 3 up to 7 day after tumor transplantation.
[0100] Group 4—10 mice replanted with Erlich carcinoma. Mice were intraperitoneally injected with DNase at 0.5 mg / kg dose in 200 ml of phosphate buffer four times a day starting from 3 up to 7 day after tumor transplantation.
[0101] Results of experiments were estimated according to tumor's growth inhibition (TG...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com