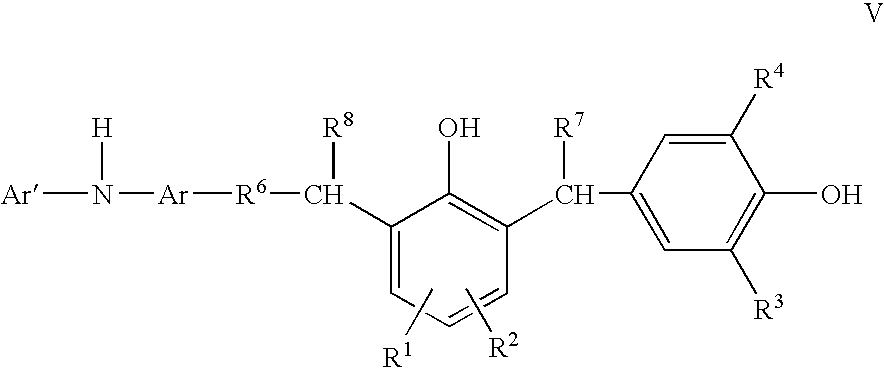
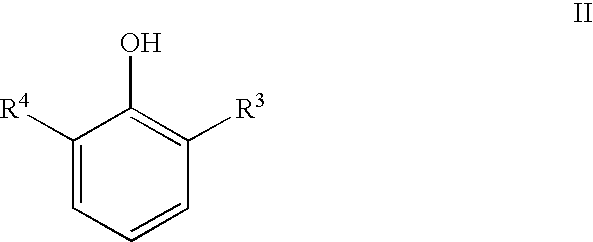
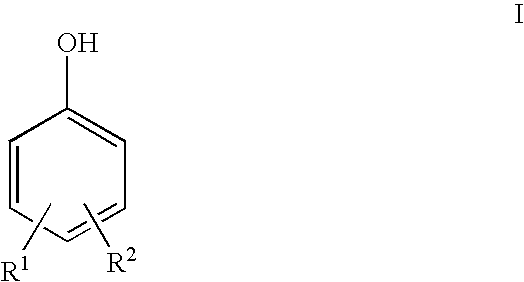Compounds and methods of making the compounds
a technology of compounds and compounds, applied in the field of antioxidants and processes for making antioxidants, can solve the problems of lubricating oils used in automobiles or trucks that are subjected to a demanding environment during use, and achieve the effect of reducing the amount of oxidation of oil and reducing the oxidation of lubricating oil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0052]A 2-liter 4-neck flask was equipped with a nitrogen atmosphere and reflux condenser. The flask was charged with 950 g of (polyisobutenyl)phenol and a few drops of a defoamer. With agitation, the contents in the flask were heated to 80° C. and 138 g of N-phenyl-phenylenediamine (NPPDA) was added in portions, followed by dropwise addition of 60.8 g of 37% aqueous solution of formaldehyde over approximately 90 min. The reaction was then held at 80° C. for 4 hrs and then at reflux for 4 hrs. The reaction mixture was vacuum stripped at 110° C. The product was diluted with 336 g of process oil and then filtered over Celite. A total of approx. 1307 g of product thus obtained had TAN (total acid number) of 28.6 and % N=1.52.
[0053]552.6 g of the above product and 61.8 g of 2,6-ditertbutylphenol were charged to a 1-liter flask equipped with nitrogen atmosphere and reflux condenser. With agitation, the contents in the flask were heated to 60° C. and 1.5 g of dimethylaminopropylamine (DMA...
example 2
[0054]A 1-liter 4-neck flask was charged with 314.4 g of 4-dodecylphenol and 247.2 g of 2,6-ditertbutylphenol and equipped with nitrogen atmosphere and reflux condenser. With agitation, the contents in the flask were heated to 60° C. and 6 g of DMAPA catalyst was added followed by dropwise addition of 97.2 g of 37% aqueous solution of formaldehyde over approximately 20 min. The reaction was then held at 60° C. for 1 hr. Reflux condenser was replaced with a distillation condenser and the temperature then gradually raised to 150° C. A total of approximately 558 g of product thus obtained had TAN (total acid number) of 122.4.
[0055]344 g of the above product, 321.7 g of process oil and few drops of a defoamer were charged to a 1-liter flask equipped with nitrogen atmosphere and reflux condenser. With agitation, the contents in the flask were heated to 80° C. and 128.8 g of N-phenyl-phenylenediamine (NPPDA) was added in portions, followed by dropwise addition of 60.8 g of 37% aqueous sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| viscosity index | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com