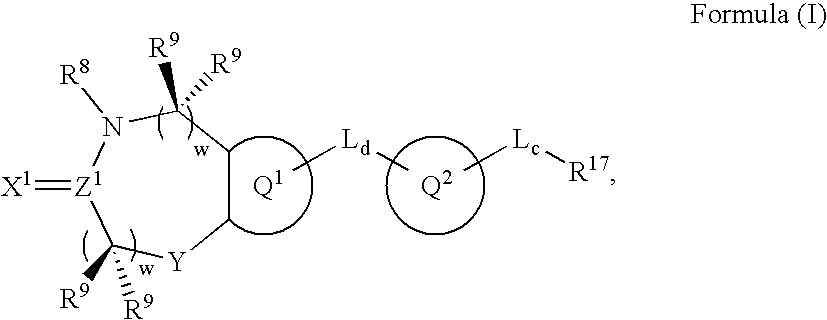
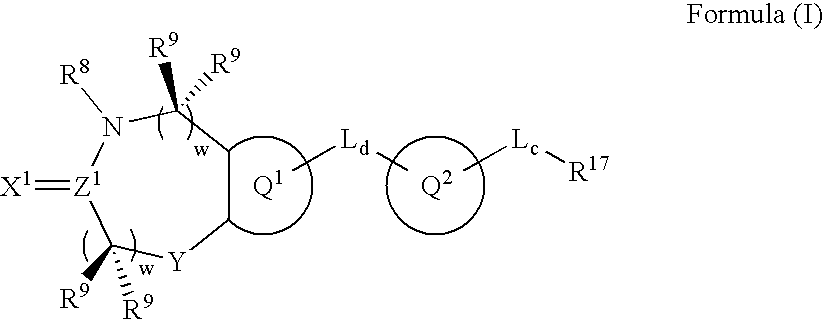
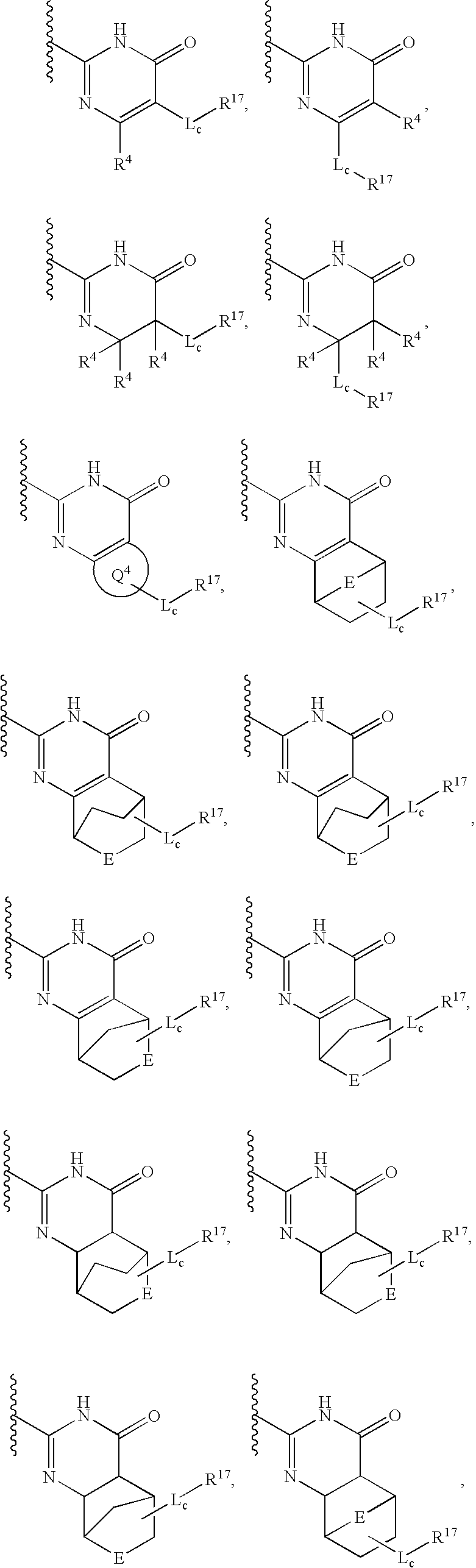
Metalloprotease inhibitors containing a heterocyclic moiety
a heterocyclic moiety and metaloprotease technology, applied in the field of new heterocyclic moiety containing pharmaceutical agents, can solve the problems of effective mmp inhibiting compounds, and achieve the effect of treating or preventing metalloprotease—especially
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparative Example 2
Step A
5-Amino-3-phenylisoxazole (995 mg) was suspended in dry pyridine (20 mL) and ethyloxalyl chloride (830 μL) was added under ice cooling. The ice bath was removed and the mixture was stirred for 2 d at room temperature and evaporated. The residue was diluted with ethyl acetate and subsequently washed with 10% aqueous citric acid and brine, dried and concentrated. The residue was recrystallized from ethyl acetate / cyclohexane to give the title compound (969 mg, 60%) as colourless needles. [MH]+=261.
Step B
The title compound from Step A above (548 mg) and PtO2 (115 mg) were suspended in EtOH (20 mL) and hydrogenated at atmospheric pressure for 2 h at 50° C., filtered over celite and refluxed for further 2 h, evaporated and purified by flash chromatography (cyclohexane / ethyl acetate 6:4 to 4:6) to afford the title compound as a colourless solid. [MH]+=245.
Step C
To a solution of the title compound from Step B above (105 mg) in THF (20 mL) was added 1M aqueous LiOH...
examples 3a to 3b
Preparative Examples 3a to 3b
Following a similar procedure as described in the Preparative Example 3, except using the aminoalcohols indicated in Table I.3 below, the following compounds were prepared.
TABLE I.3Prep. Ex. #aminealcoholproductyield3an.d.[MH]+ = 246 / 483b19%[MH]+ = 246 / 48
Preparative Example 4
Step A
A suspension of the title compound from the Preparative Example 3, Step B (567 mg) and CuCN (230 mg) in dry N-methyl-pyrrolidin-2-one (15 mL) was degassed under Argon and heated under microwave irradiation to 200° C. for 2 h. The mixture was concentrated, diluted with 1N HCl (100 mL) and extracted with EtOAc (200 mL). The organic layers were washed with H2O (2×200 mL) and brine (200 mL), dried (MgSO4), filtered, absorbed on silica and purified by flash chromatography (cyclohexane / ethyl acetate 7:3 to 6:5) to give the title compound as a colourless solid. [MH]+=211.
Step B
To an ice cooled solution of the title compound from Step A above in dry MeOH (20 mL) were added di-tert-buty...
examples 4a to 4d
Preparative Examples 4a to 4d
Following a similar procedure as described in the Preparative Example 4, Step A except using the educt indicated in Table I.4 below, the following compounds were prepared.
TABLE I.4Prep. Ex. #eductproductyield4an.d.[MH]+= 1934b93%[MH]+ = 1934cn.d.[MH]+ = 1934d55%[MH]+ = 211
Preparative Examples 5a to 5f
Following a similar procedure as described in the Preparative Example 4, Step B except using the educt indicated in Table I.5 below, the following compounds were prepared.
TABLE I.5Prep. Ex. #eductproductyield5a32% (3 steps)[MNa]+ = 3195b56%[MNa]+ = 3195c28% (2 steps)[MNa]+ = 3195dn.d.[MNa]+ = 3255e58%[MNa]+ = 3015f64%[MNa]+ = 337
Preparative Examples 6a to 6h
Following a similar procedure as described in the Preparative Example 4, Step C except using the educt indicated in Table I.6 below, the following compounds were prepared.
TABLE I.6Prep. Ex. #eductproductyield6aquant.[M − Cl]+ = 1976bquant.[M − Cl]+ = 1956cquant.[M − Cl]+ = 1976dquant.[M − Cl]+ = 1976e66% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com