Pd-1 antibodies in combination with a cytokine-secreting cell and methods of use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immune Response Following Vaccination With a GM-CSF-Secreting Immunotherapy Combined With Administration of Anti-PD-1 Antibody
[0175]Immune responses were measured as increases in tumor antigen-specific T-cells. Such T-cells can be identified by immunological monitoring methods, as described above, including (A) tetramer staining, (B) in vivo CTL activity, and (C) the induction of intracellular IFN-gamma expression following stimulation with a tumor-specific peptide or tumor cells.
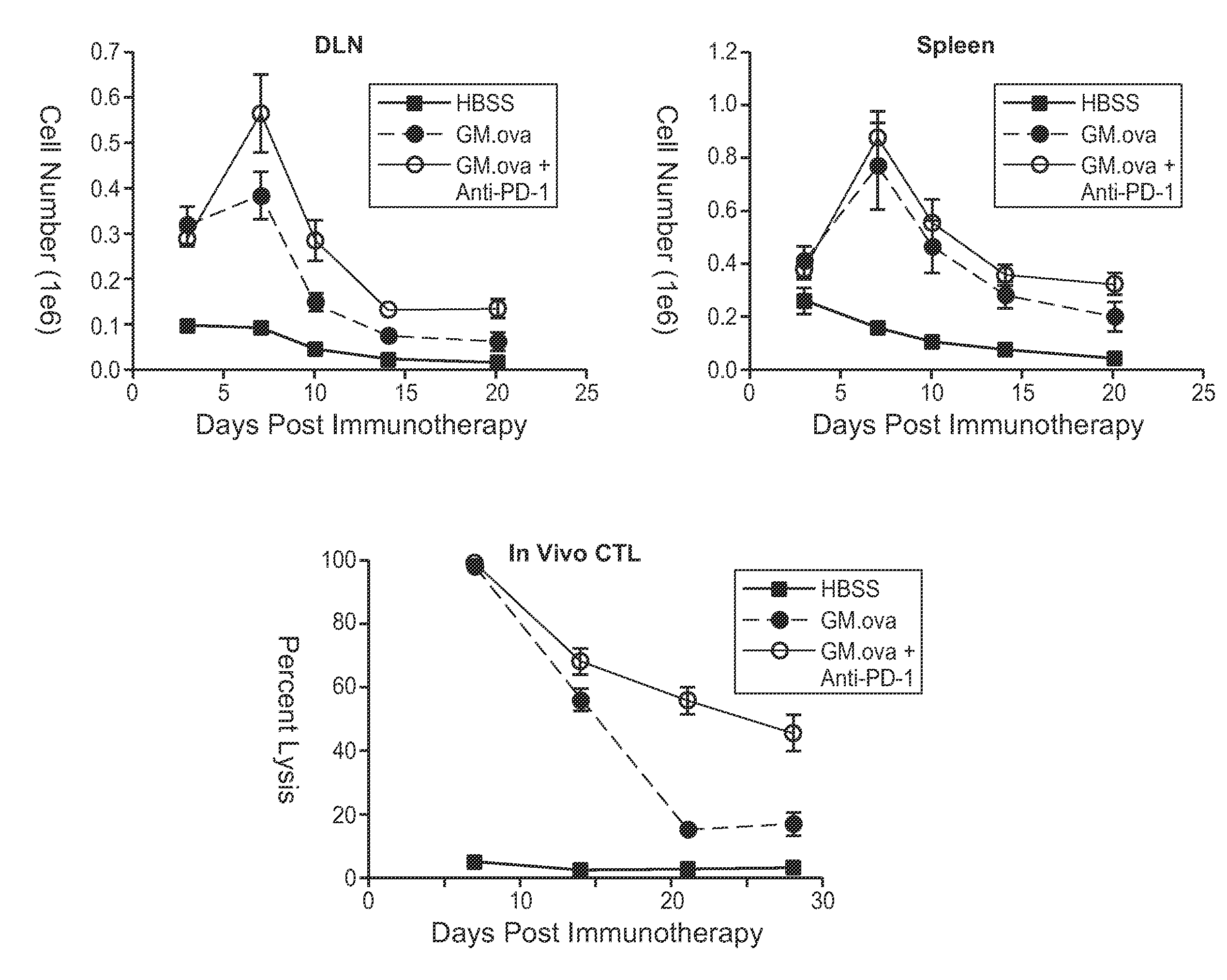
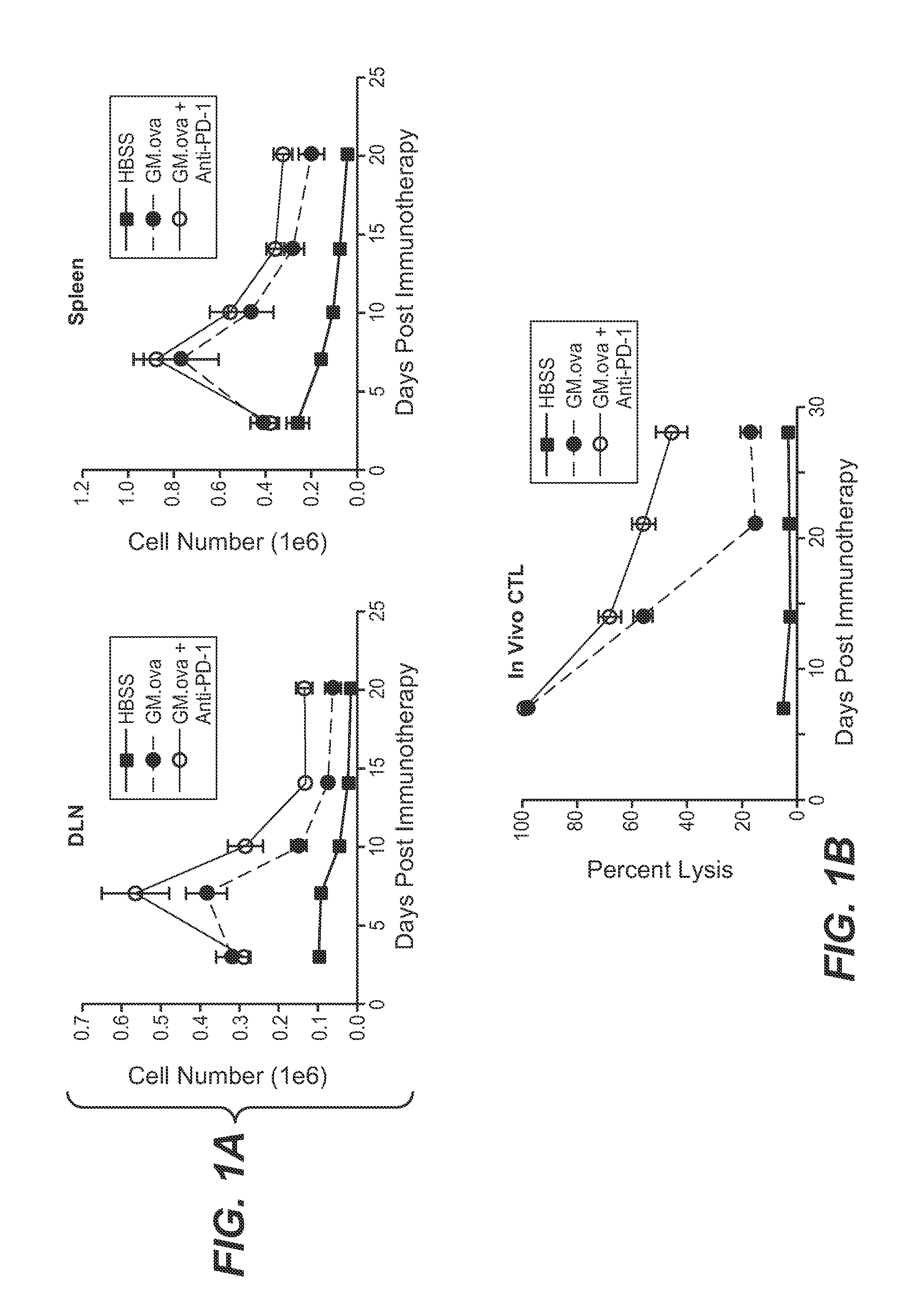
[0176]FIG. 1A illustrates the anti-tumor T-cell response induced by administration of GM-CSF-secreting B16F10 cells alone and in combination with an anti-PD-1 antibody, as determined by tetramer staining. On day 0, 1×106 OT-1 transgenic T-cells were adoptively transferred into tumor-bearing C57BL / 6 mice which were challenged on day −1 with 2×105 live B16 cells transduced with the surrogate antigen, ovalbumin (F10.ova). On day 3, mice were immunized with 1×106 irradiated GM-CSF-secreting B16 cells expressing...
example 2
Pro-inflammatory Cytokine Secretion Following GVAX / Anti-PD-1 Combination Immunotherapy
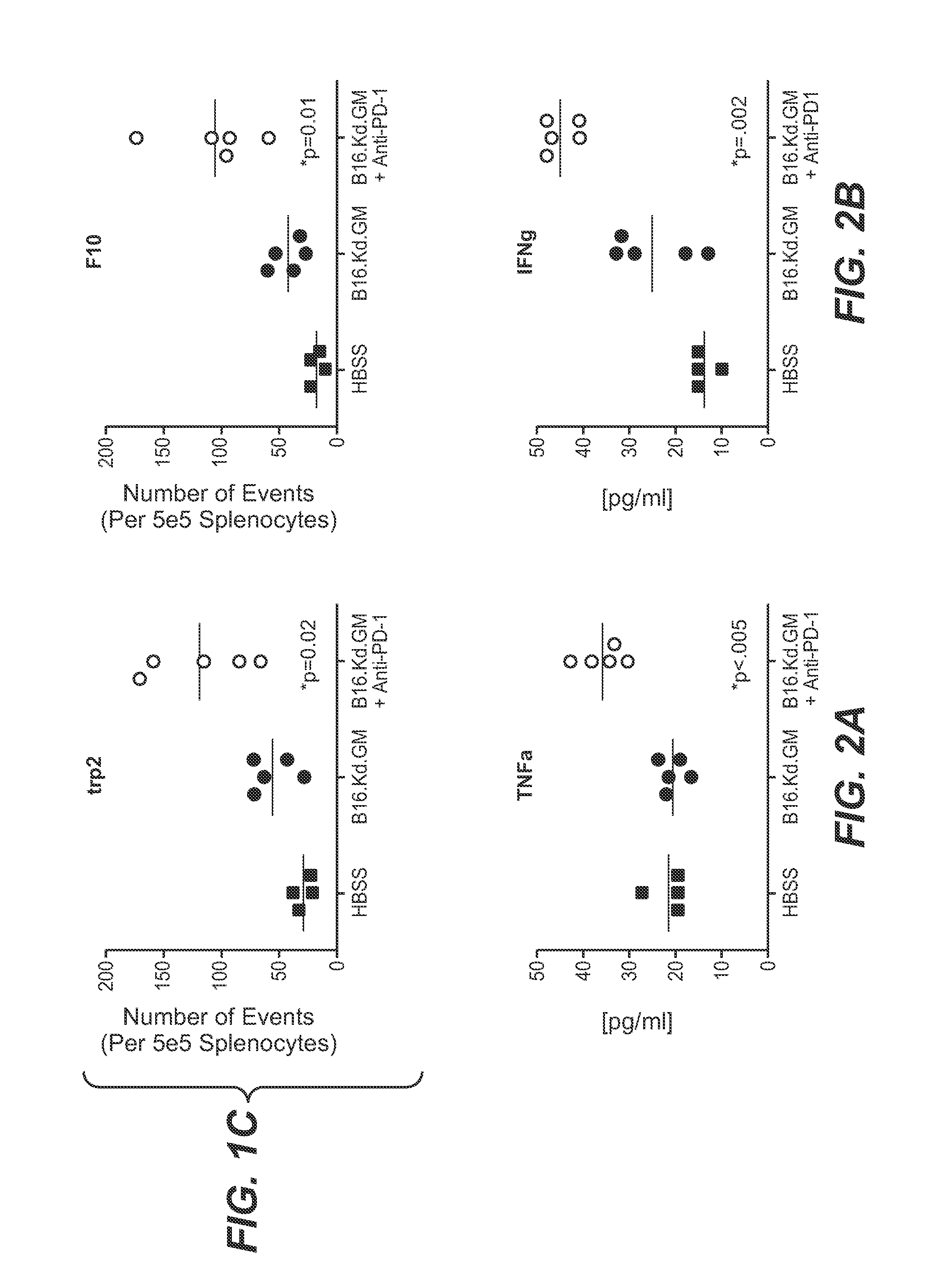
[0180]A further suggestion as to the potential utility of the combination of GM-CSF-secreting immunotherapies and anti-PD-1 antibody in eliciting an anti-tumor immune response is the production of pro-inflammatory cytokines such as TNF-alpha, IFN-gamma, IL-2, IL-5, IL-6, IL-10 and MCP-1 upon restimulation in vitro. Release of such cytokines is often used as a surrogate marker for monitoring tumor-specific immune responses following immunotherapeutic strategies designed to induce anti-tumor immunity.
[0181]FIG. 2 illustrates the secretion of pro-inflammatory cytokines following administration of GM-CSF-secreting B16F10 cells alone and in combination with an anti-PD-1 antibody. On day 0, mice were inoculated subcutaneously with live B16F10 cells. On day 3, mice were immunized with 1×106 irradiated GM-CSF-secreting B16F10 cells (B16.Kd.GM) as immunotherapy alone, or immunotherapy was followed by 200 μg...
example 3
Recruitment of T-cells into Tumors Following GVAX / Anti-PD-1 Combination Immunotherapy
[0183]FIG. 3 illustrates effector CD8 T-cell infiltration into tumors in mice treated with GM-CSF-secreting B16F10 cells alone or in combination with an anti-PD-1 antibody. High numbers of tumor infiltrating lymphocytes (TIL) within tumors have been shown to correlate with overall therapeutic benefit (Dunn et al., Nat. Immunol. 3(11):991-8, 39 (2002); Smyth et al., Nat Immunol. 2(4):293-9 (2001). Thus, the extent of infiltrating lymphocytes in tumors of B16.Kd.GM-treated animals compared to animals treated with the combination therapy was examined. On day 0, mice were inoculated subcutaneously with live B16F10 cells. On day 3, 1×106 irradiated GM-CSF-secreting B16F10 cells (B16.Kd.GM) were injected as immunotherapy alone, or with 200 μg and 100 μg of anti-PD-1 antibody on days 3 and 4, respectively, as combination therapy. At the indicated timepoints, tumors from mice were excised, digested and stai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com