Ophthalmic And Otic Compositions Of Facially Amphiphilic Polymers And Oligomers And Uses Thereof
a technology of amphiphilic polymers and compositions, applied in the field of amphiphilic antimicrobial compositions of facially amphiphilic polymers and oligomers, can solve the problems of bacterial drug resistance, determinants, and virtually untreatable current antimicrobial agents, and achieve the effect of treating or preventing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antimicrobial Activity
Minimum Inhibitory Concentrations
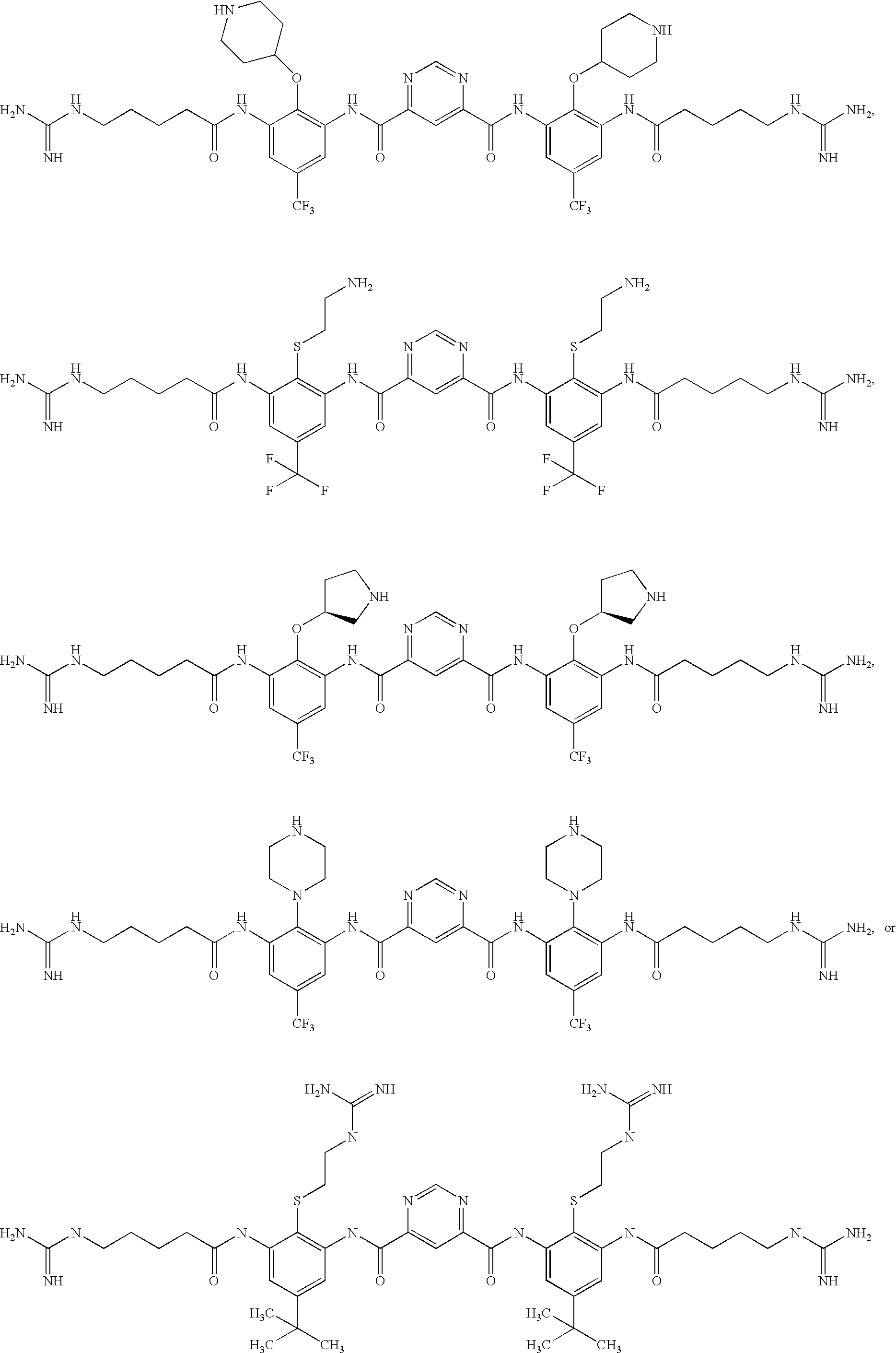
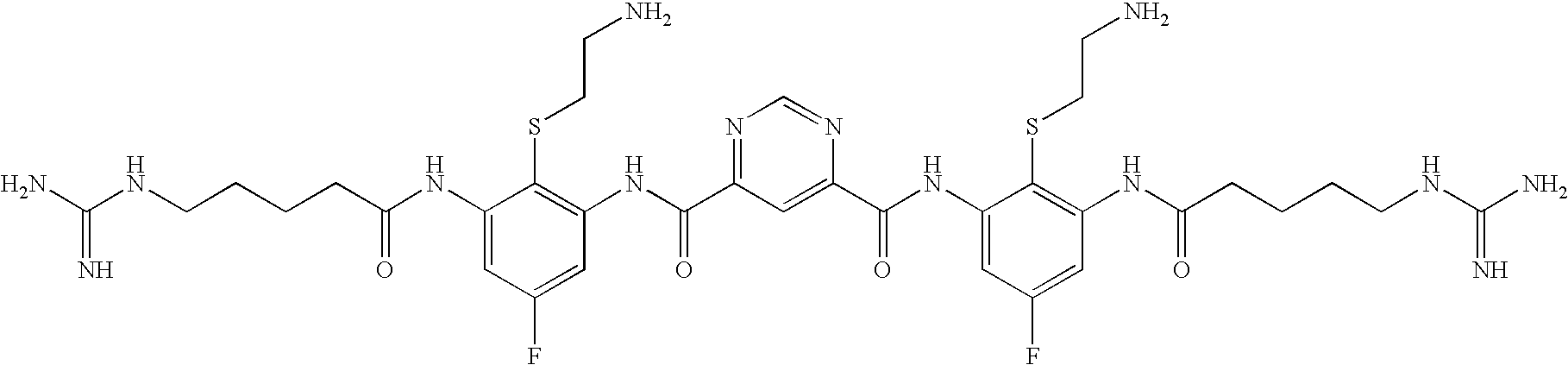
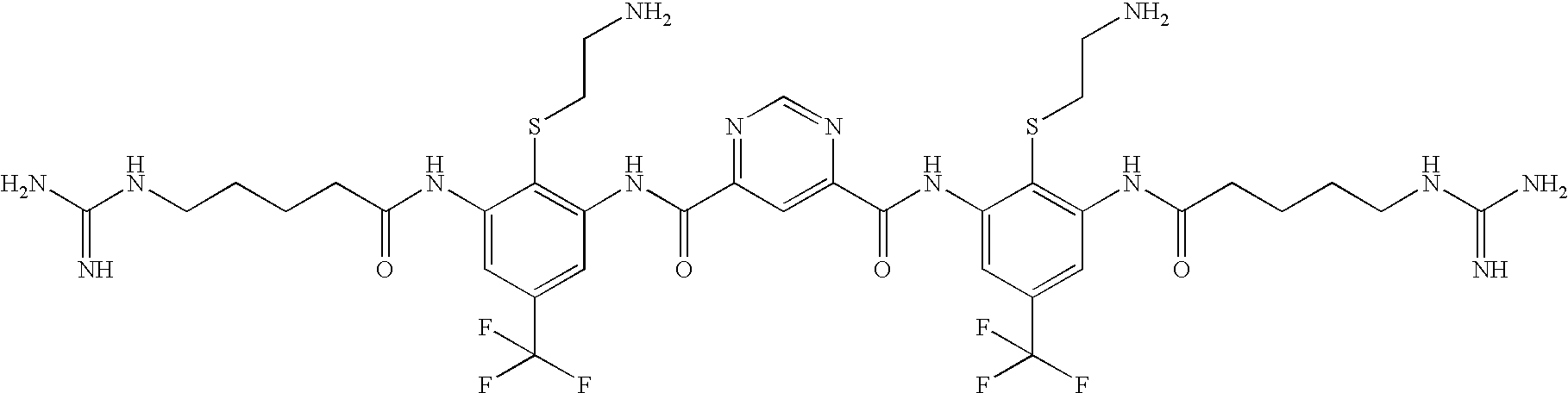
[0832]The following three oligomers of the invention were screened for antimicrobial activity against a number of clinically relevant ocular pathogens.
[0833]Minimum Inhibitory Concentrations (MIC) of each of the 3 oligomers were determined using standard procedures for clinical ocular isolates of Ciprofloxacin Susceptible (CS) S. aureus (CSSA) (n=27), Ciprofloxacin Resistant (CR) S. aureus (CRSA) (n=28), CS S. epidermidis (CSSE) (n=26), CR S. epidermidis (CRSE) (n=26), St. pneumoniae (SP) (n=27), St. viridans group (SV), Moraxella Species (MS) (n=25), H. influenzae (HI) (n=26), P. aeruginosa (PA) (n=26), and Serratia marcescens (SM) (n=27).
[0834]The results are presented in Table 1. Data is expressed as MIC50, MIC90, in μg / ml for Oligomer 1, Oligomer 2, and Oligomer 3, respectively.
TABLE 1Oligomer 1Oligomer 2Oligomer 3MicrobialMIC50MIC90MIC50MIC90MIC50MIC90Strain(μg / ml)(μg / ml)(μg / ml)(μg / ml)(μg / ml)(μg / ml)CSSA0.1250.250.1250.250.5...
example 2
Ophthalmic Ointment Formulation
[0836]The following represents an example of a typical ophthalmic ointment formulation comprising an antimicrobial oligomer of the invention (oligomer 1 in Example 1 above).
Ophthalmic OintmentIngredientAmount (weight %)Oligomer 10.35Mineral Oil, USP2.0White petrolatum, USPq.s. 100
example 3
Ophthalmic Ointment Formulation
[0837]The following represents an example of a typical ophthalmic ointment formulation comprising an antimicrobial oligomer of the invention (oliogmer 2 in Example 1 above) and an anti-inflammatory agent.
Ophthalmic OintmentIngredientAmount (weight %)Oligomer 20.3Dexamethasone0.1Chlorobutanol, Anhydrous, NF0.5Mineral Oil, USP5.0White petrolatum, USPq.s. 100
PUM
| Property | Measurement | Unit |
|---|---|---|
| polar | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com