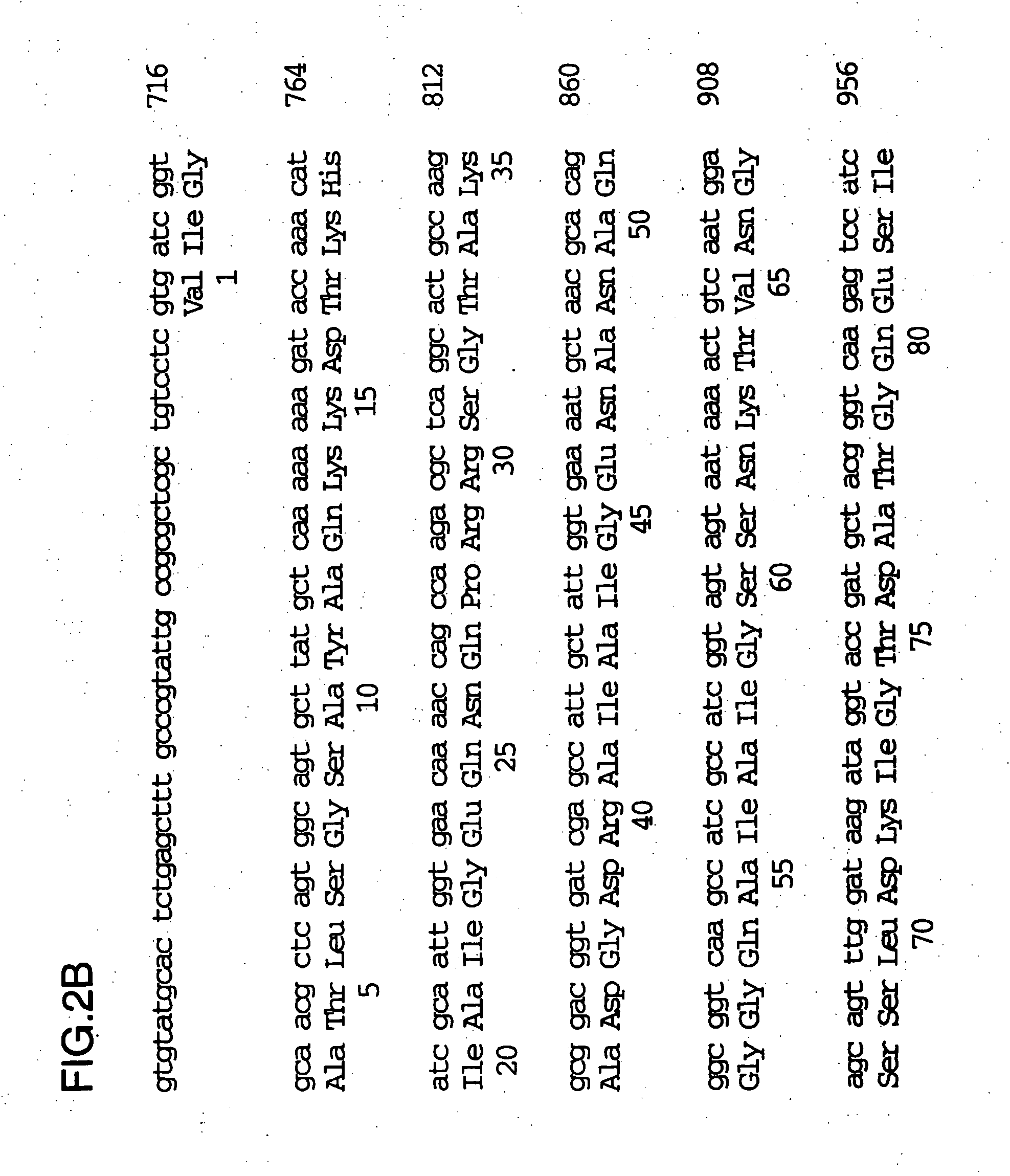
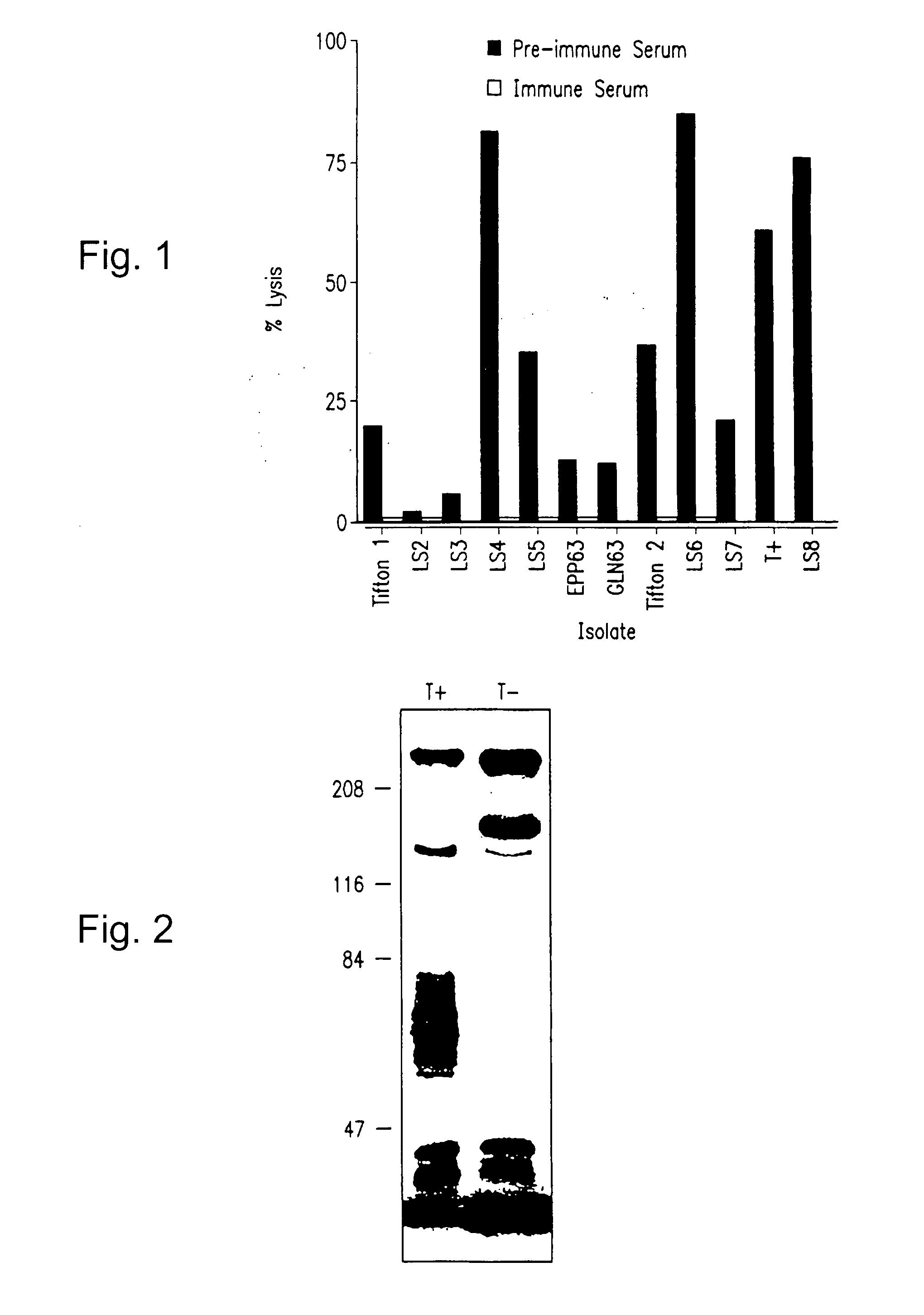
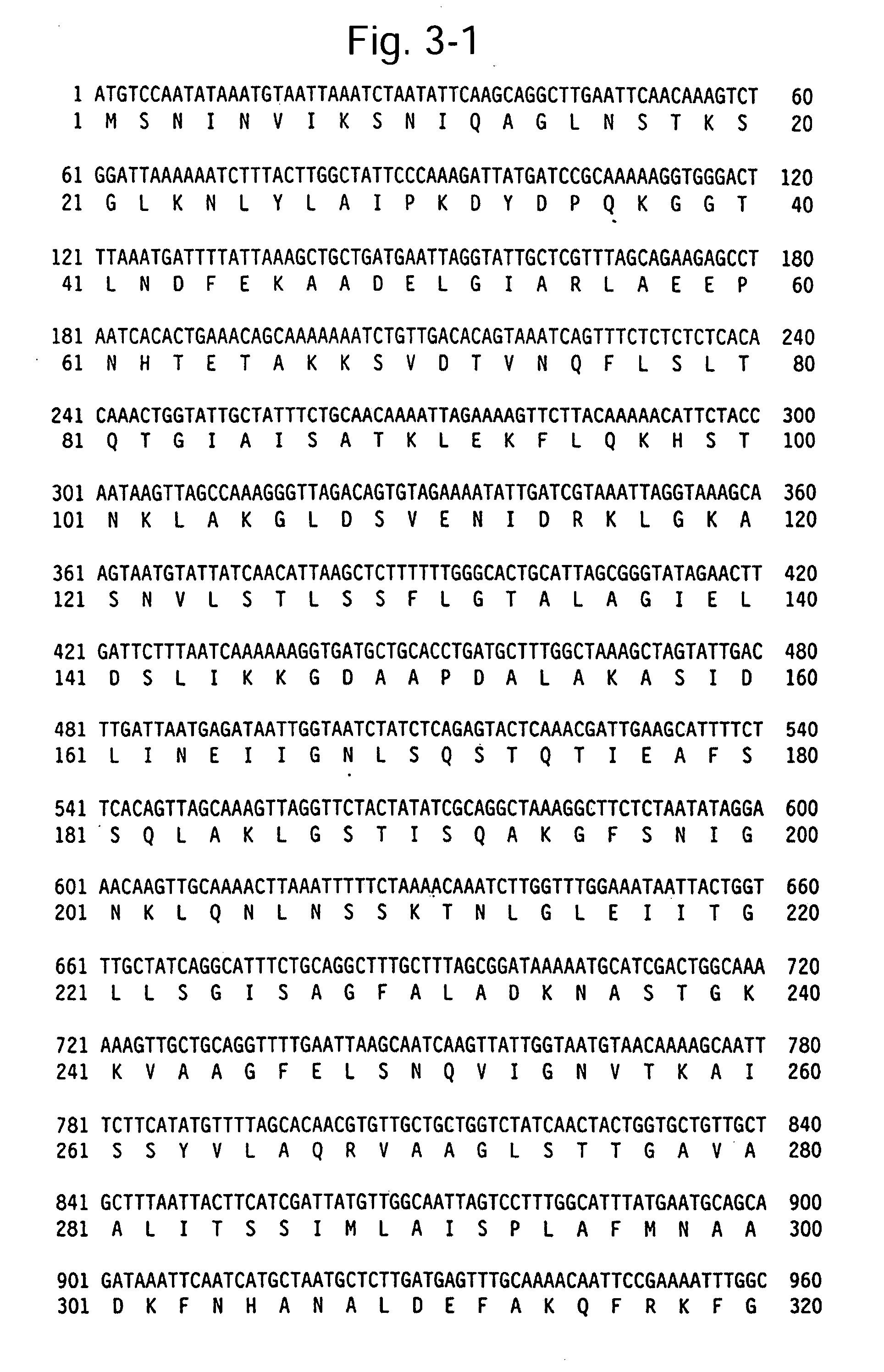
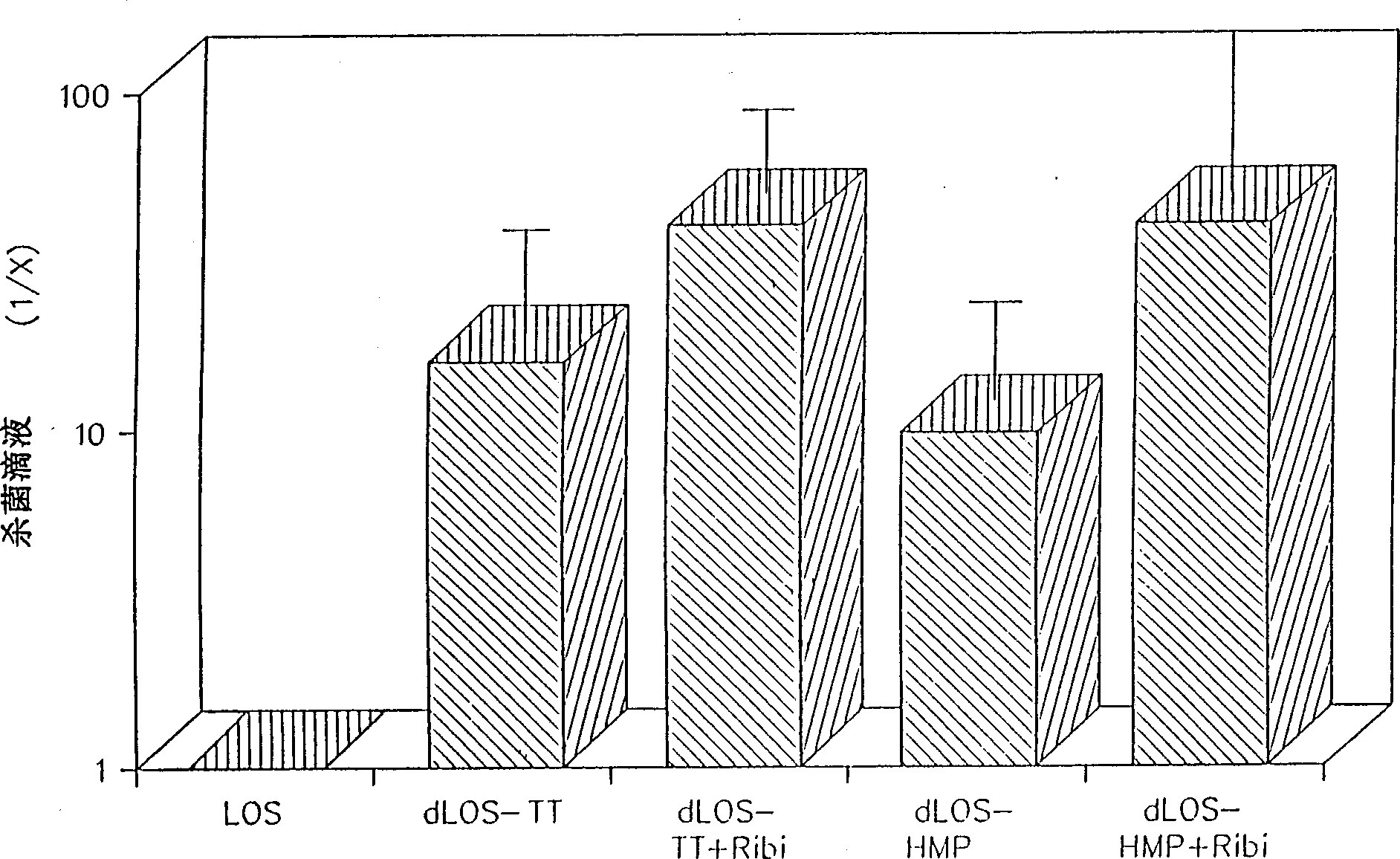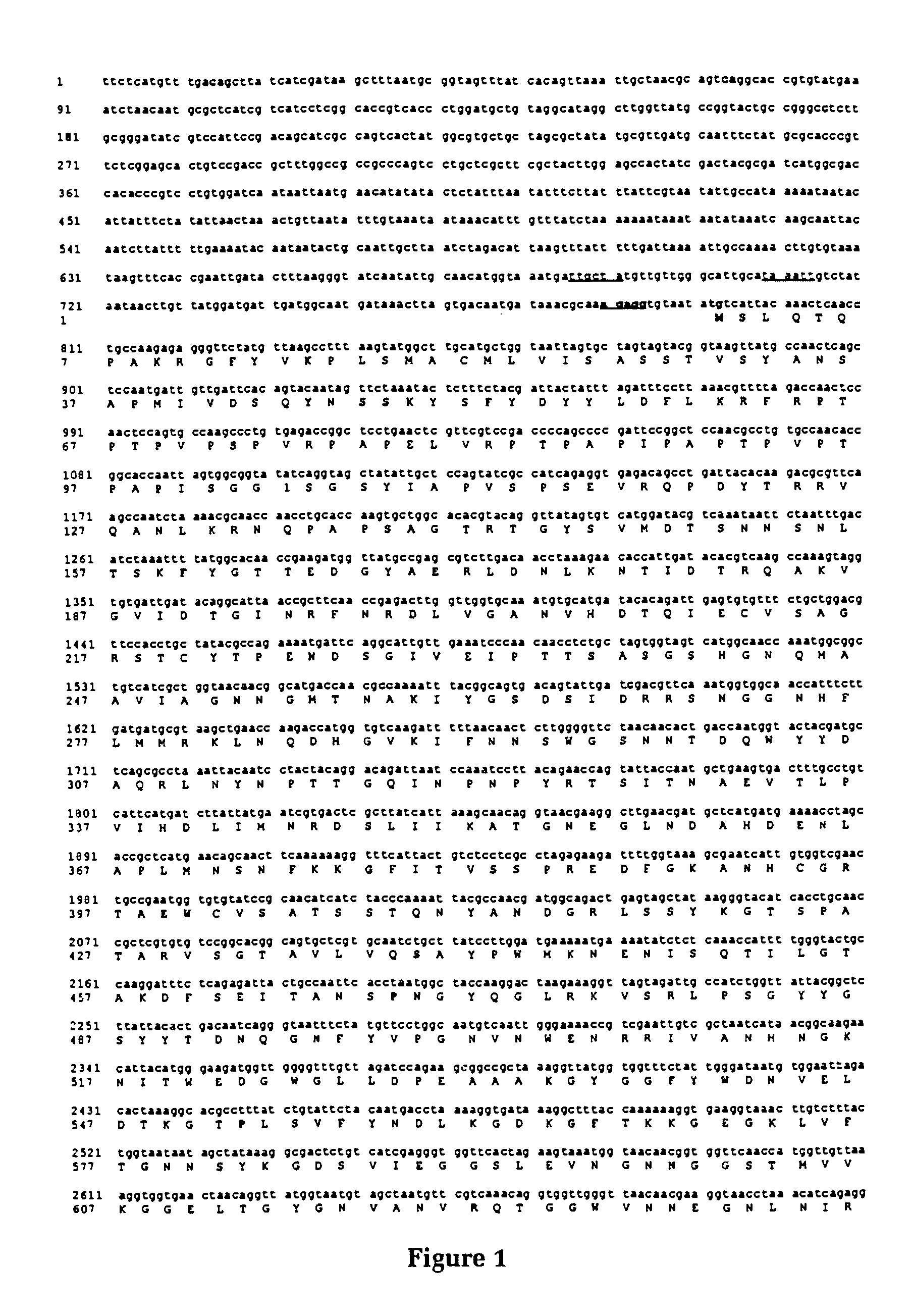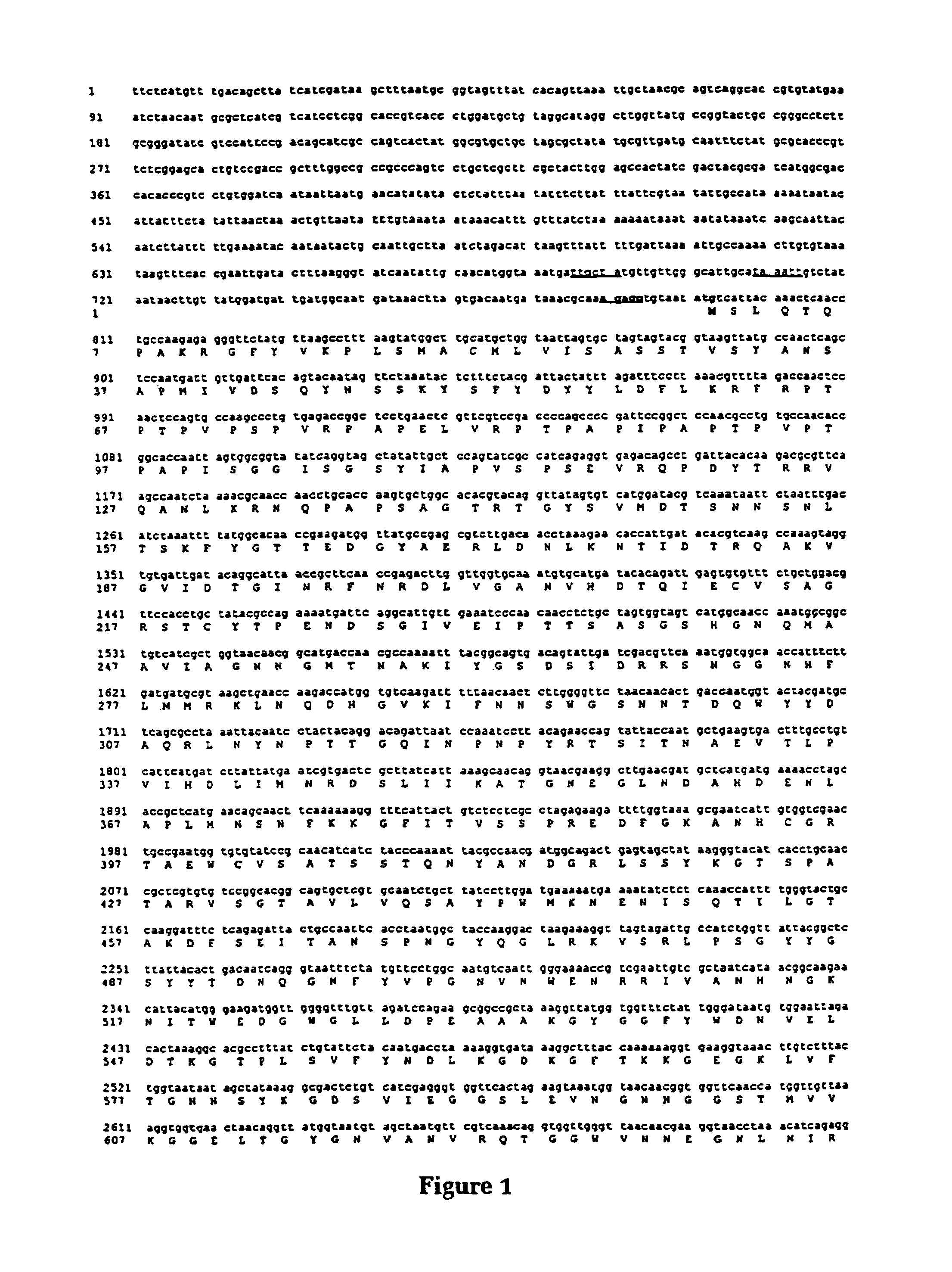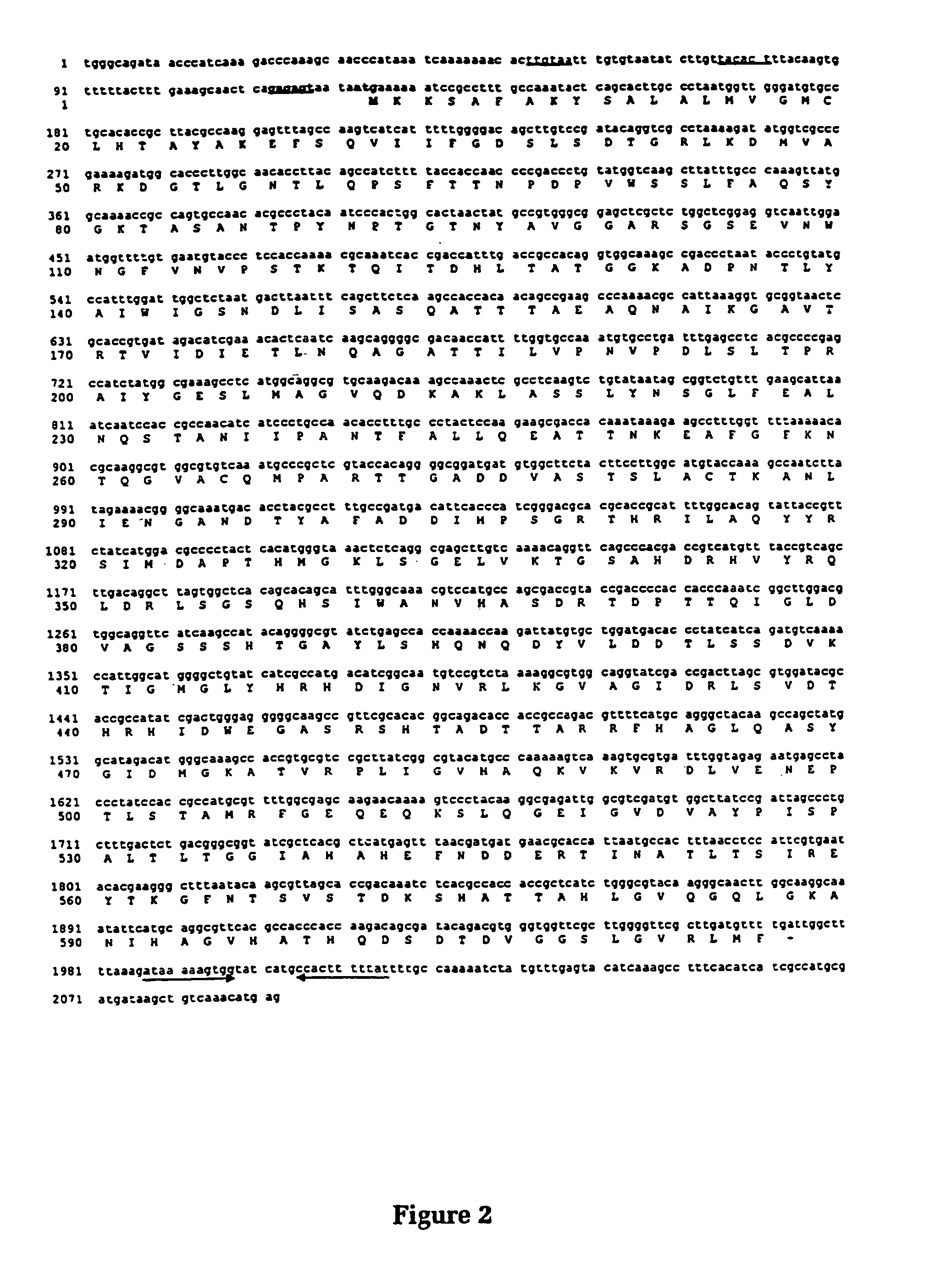Patents
Literature
30 results about "Moraxella species" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The organisms are short rods, coccobacilli, or as in the case of Moraxella catarrhalis, diplococci in morphology, with asaccharolytic, oxidase-positive, and catalase-positive properties. M. catarrhalis is the clinically most important species under this genus.
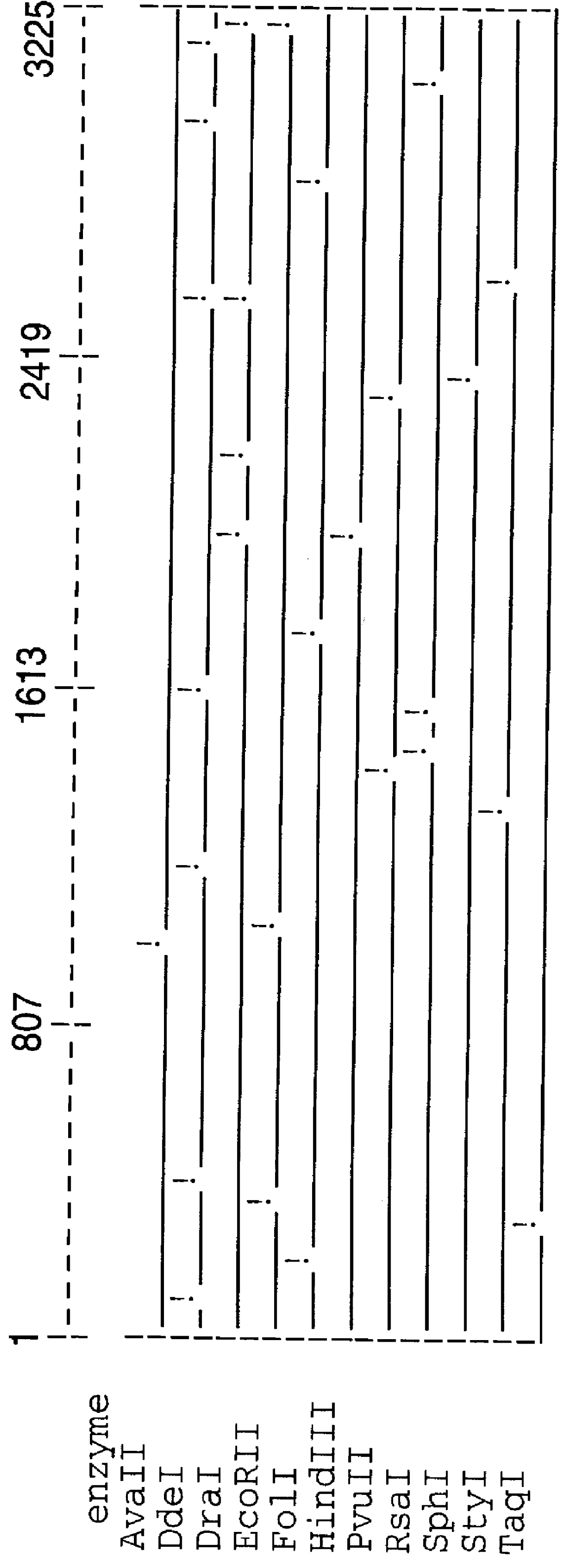
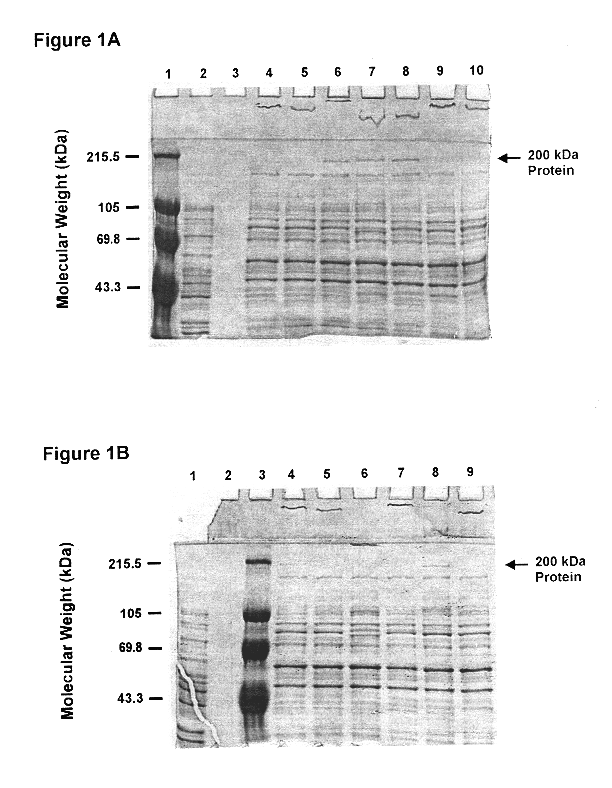
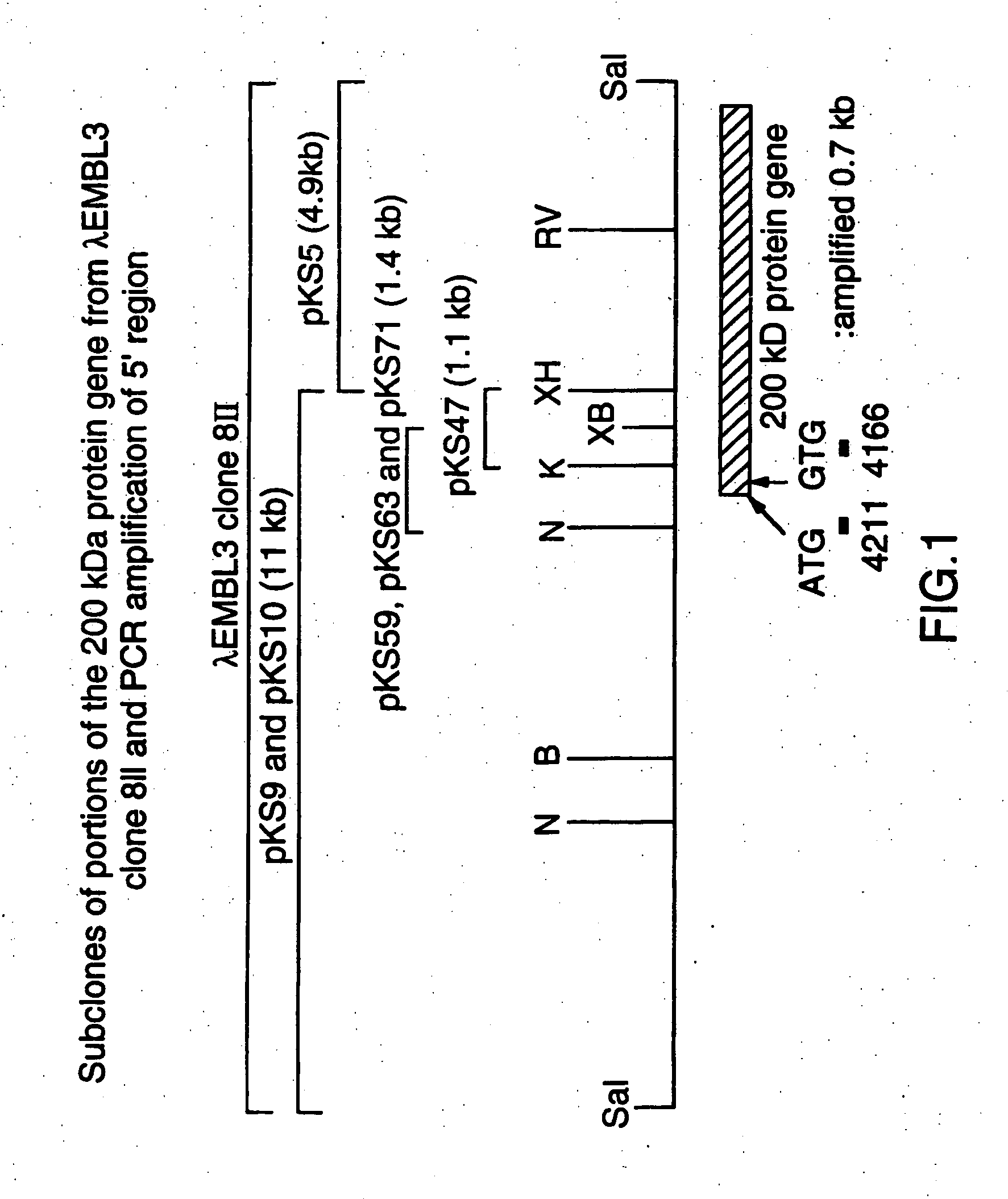
High molecular weight major outer membrane protein of moraxella
InactiveUS6335018B1Improving immunogenicitySlow and sustained releasePeptide/protein ingredientsBacteria peptidesDiseaseIn vivo
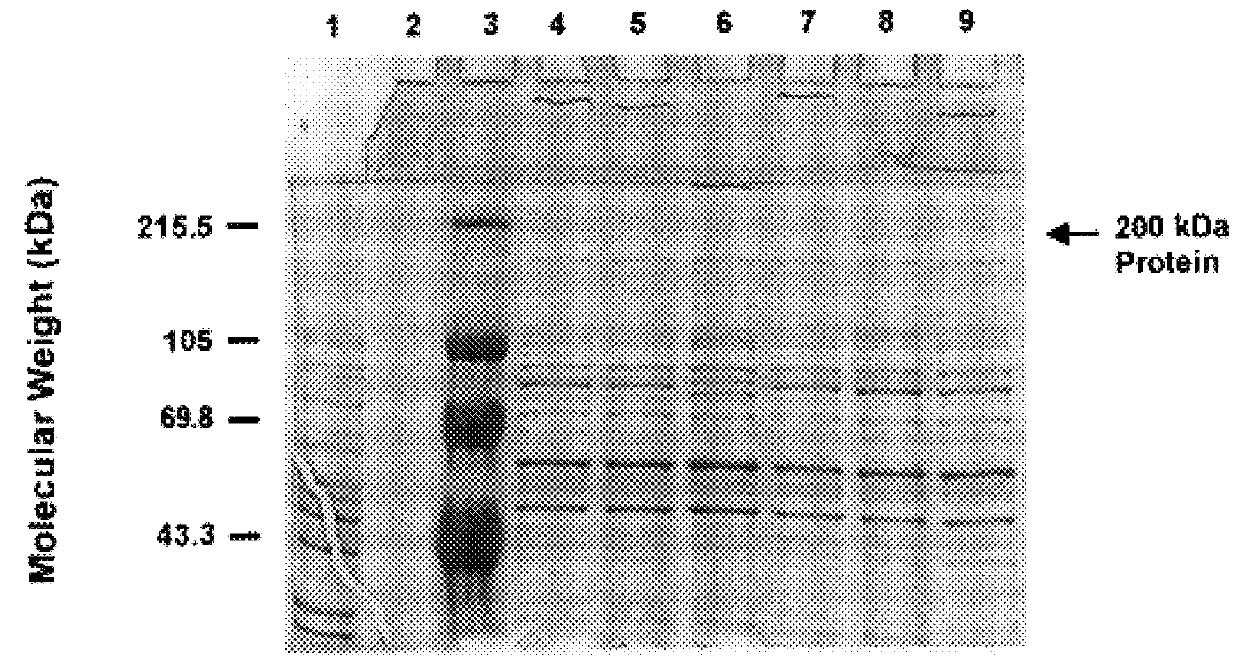
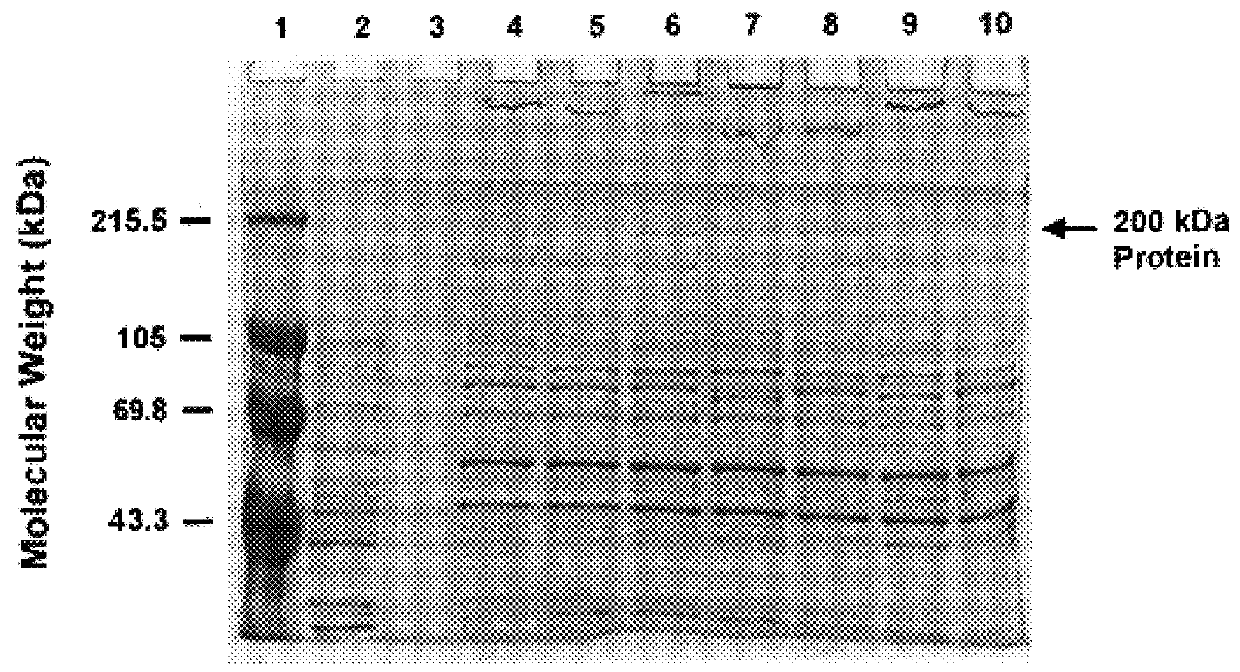
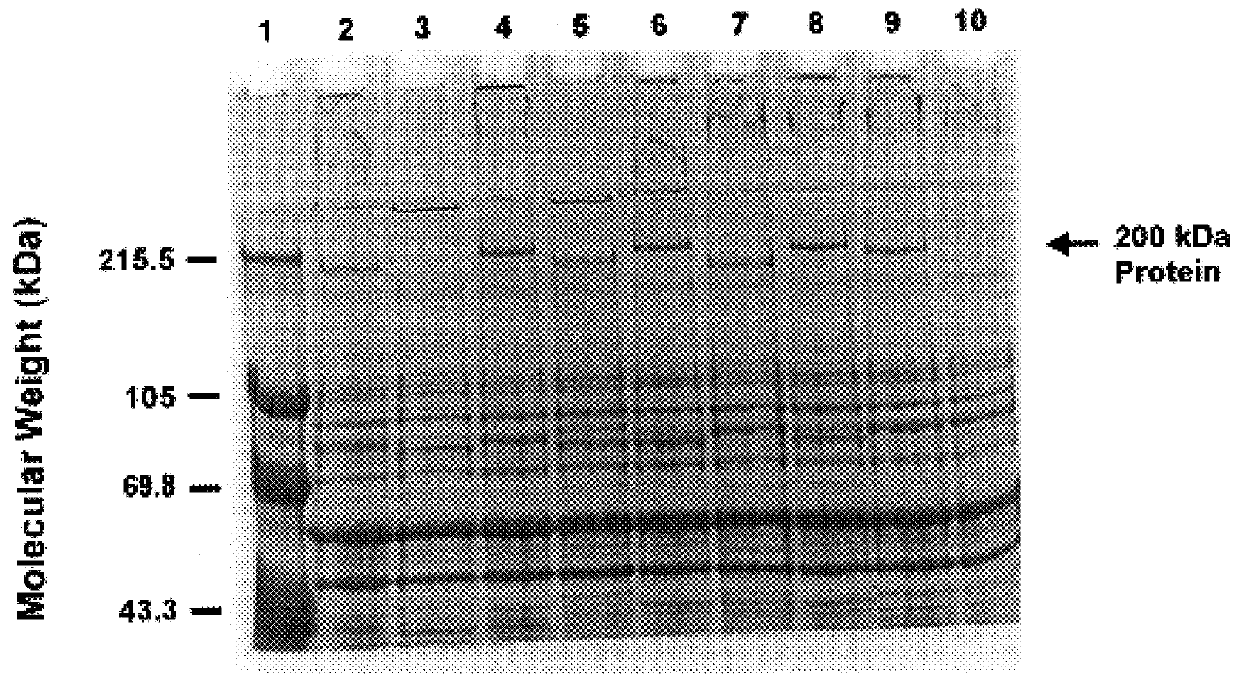
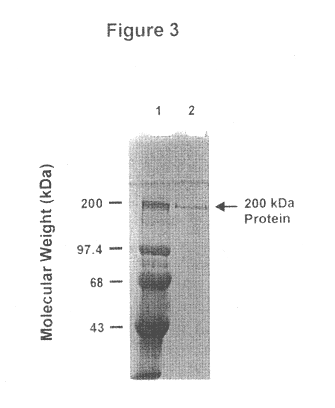
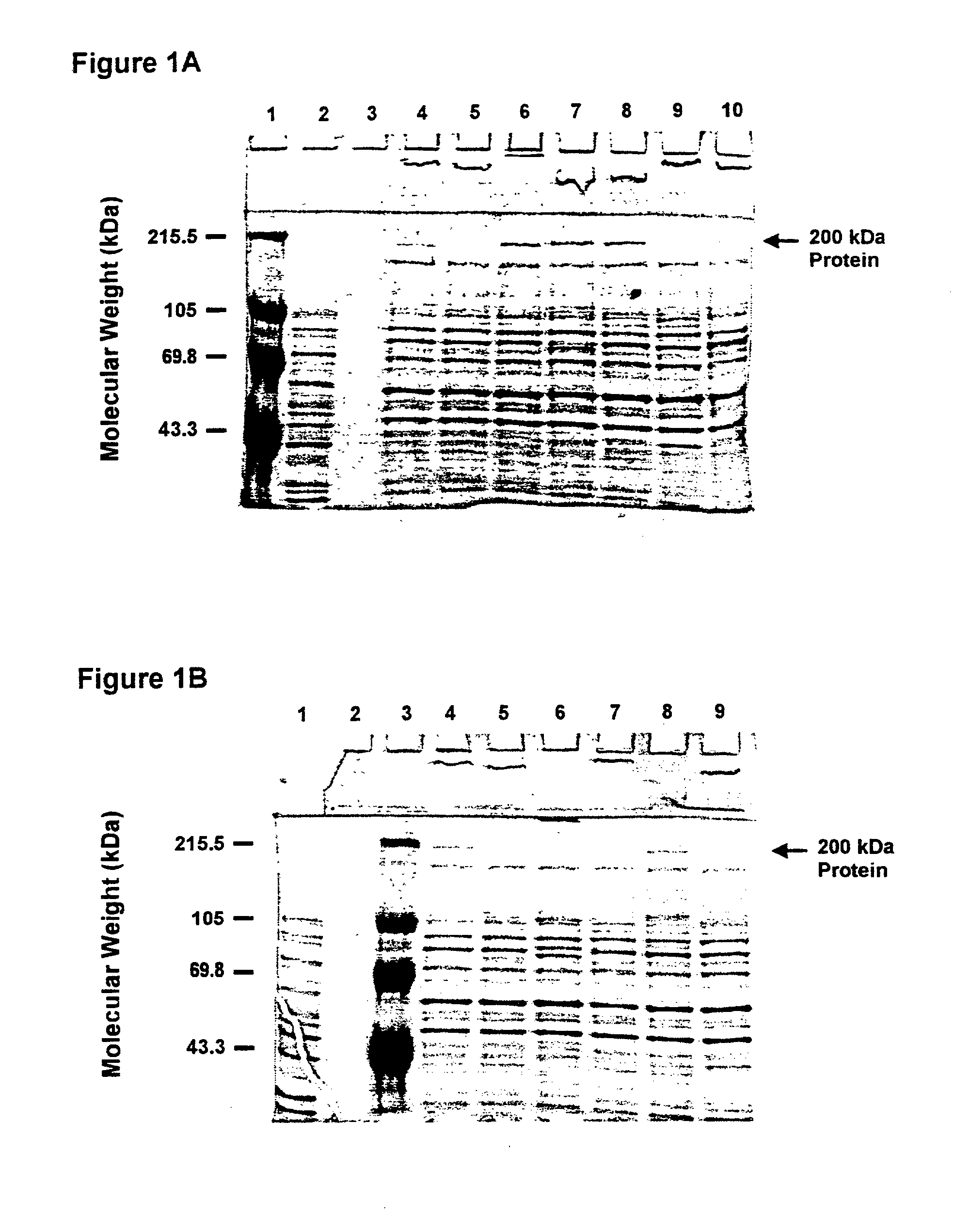
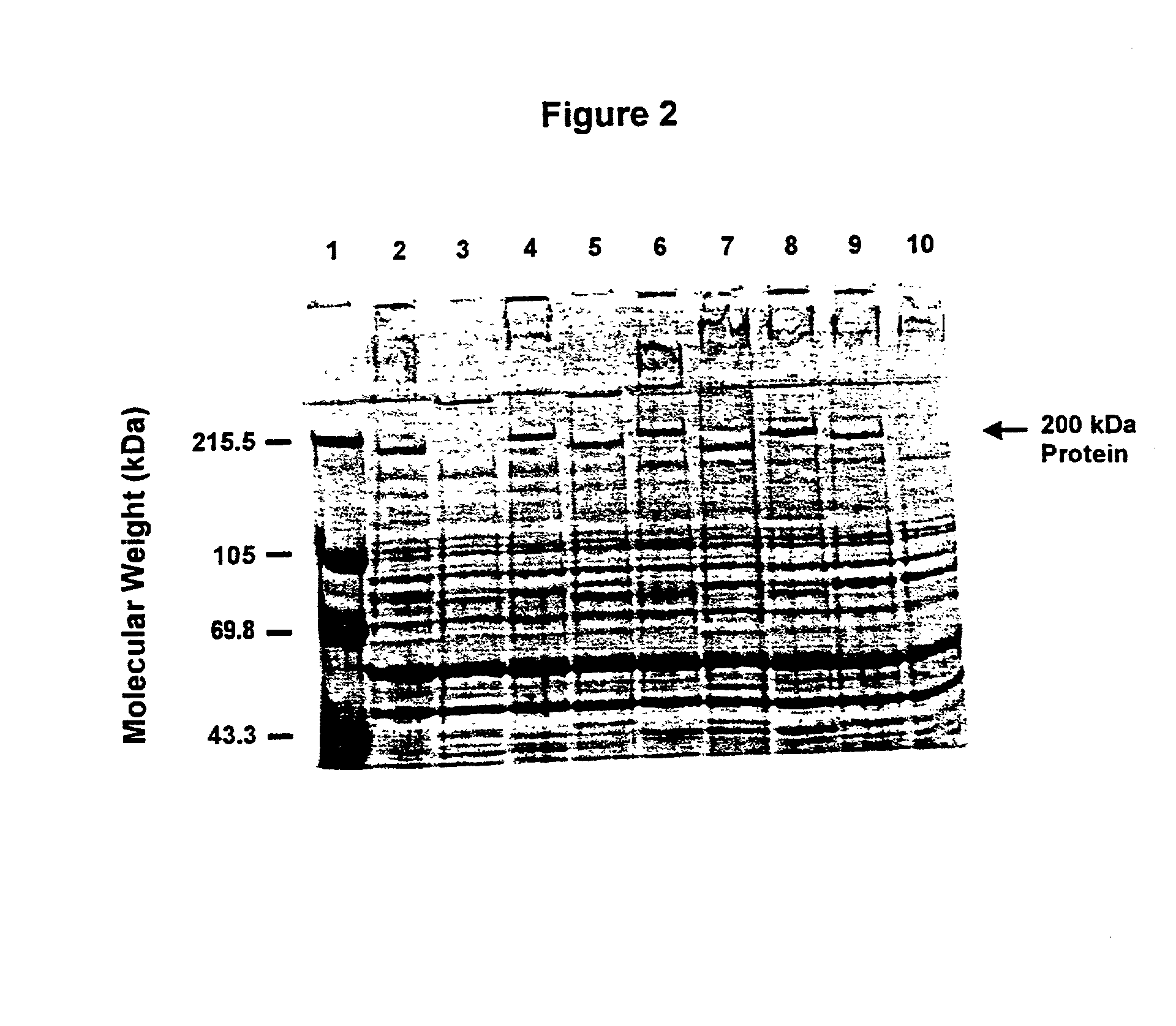
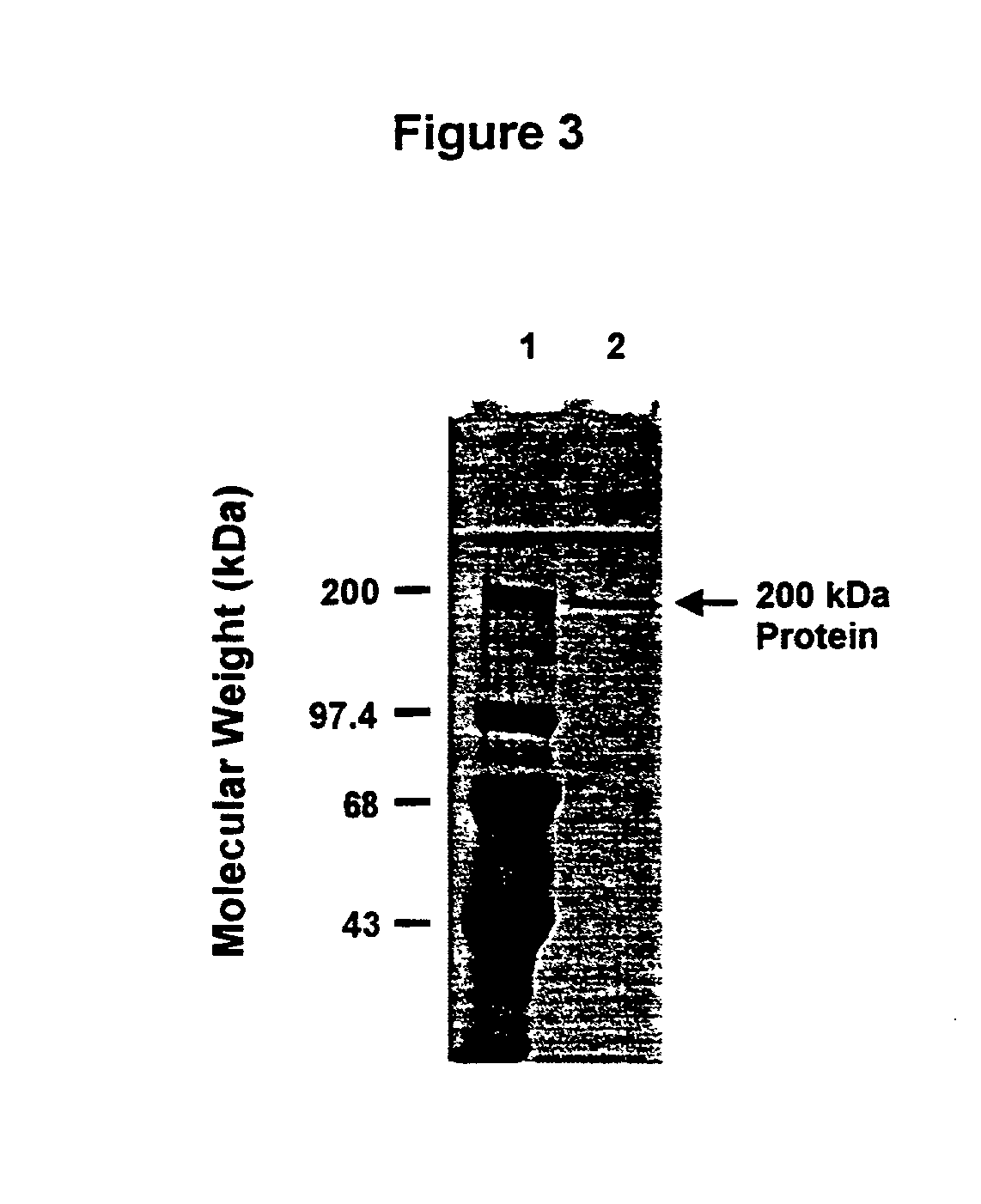
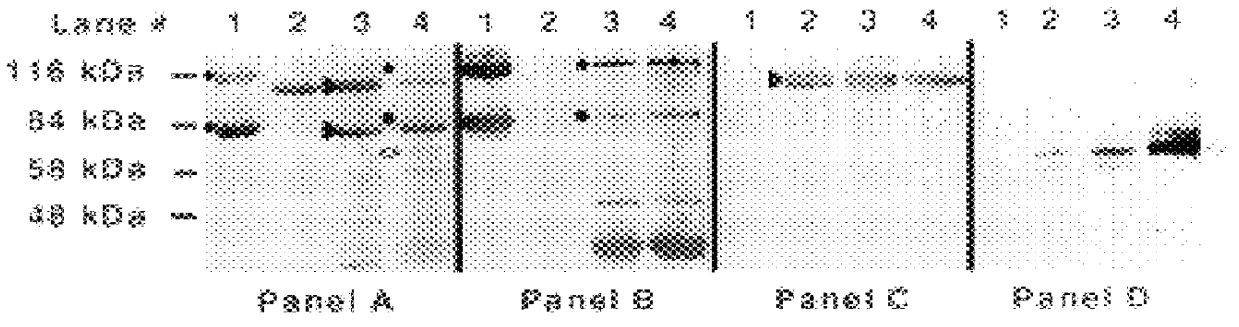
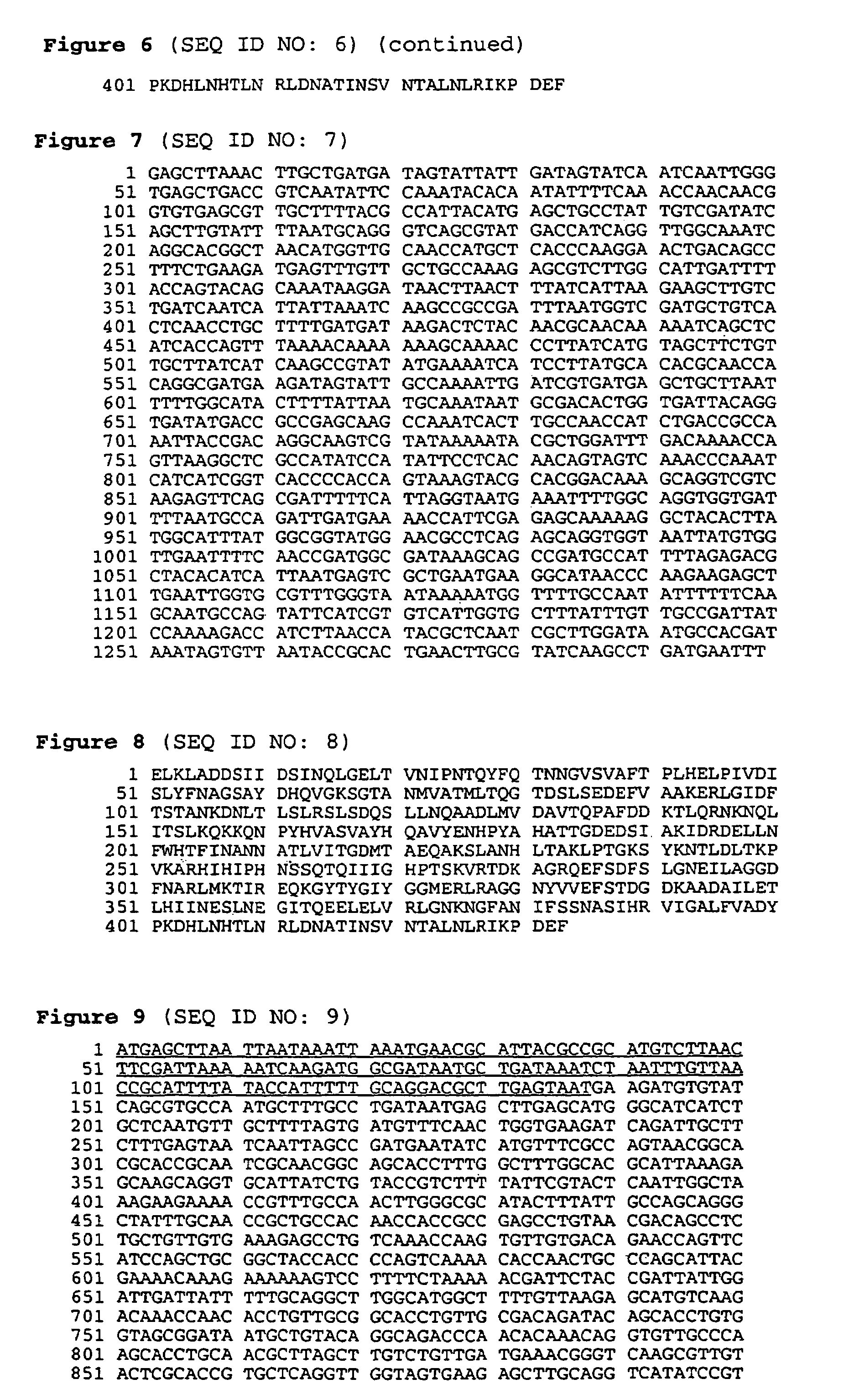
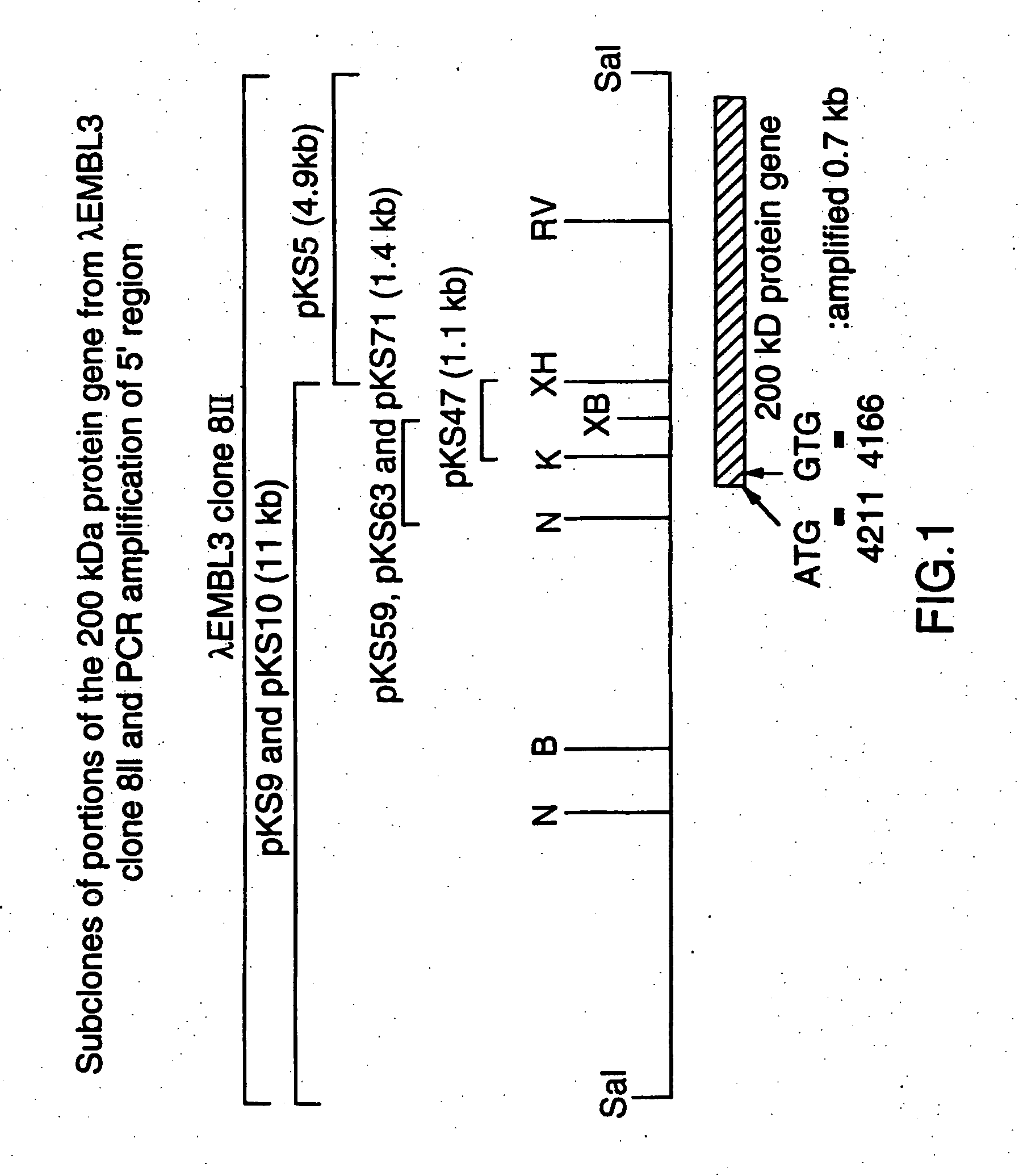
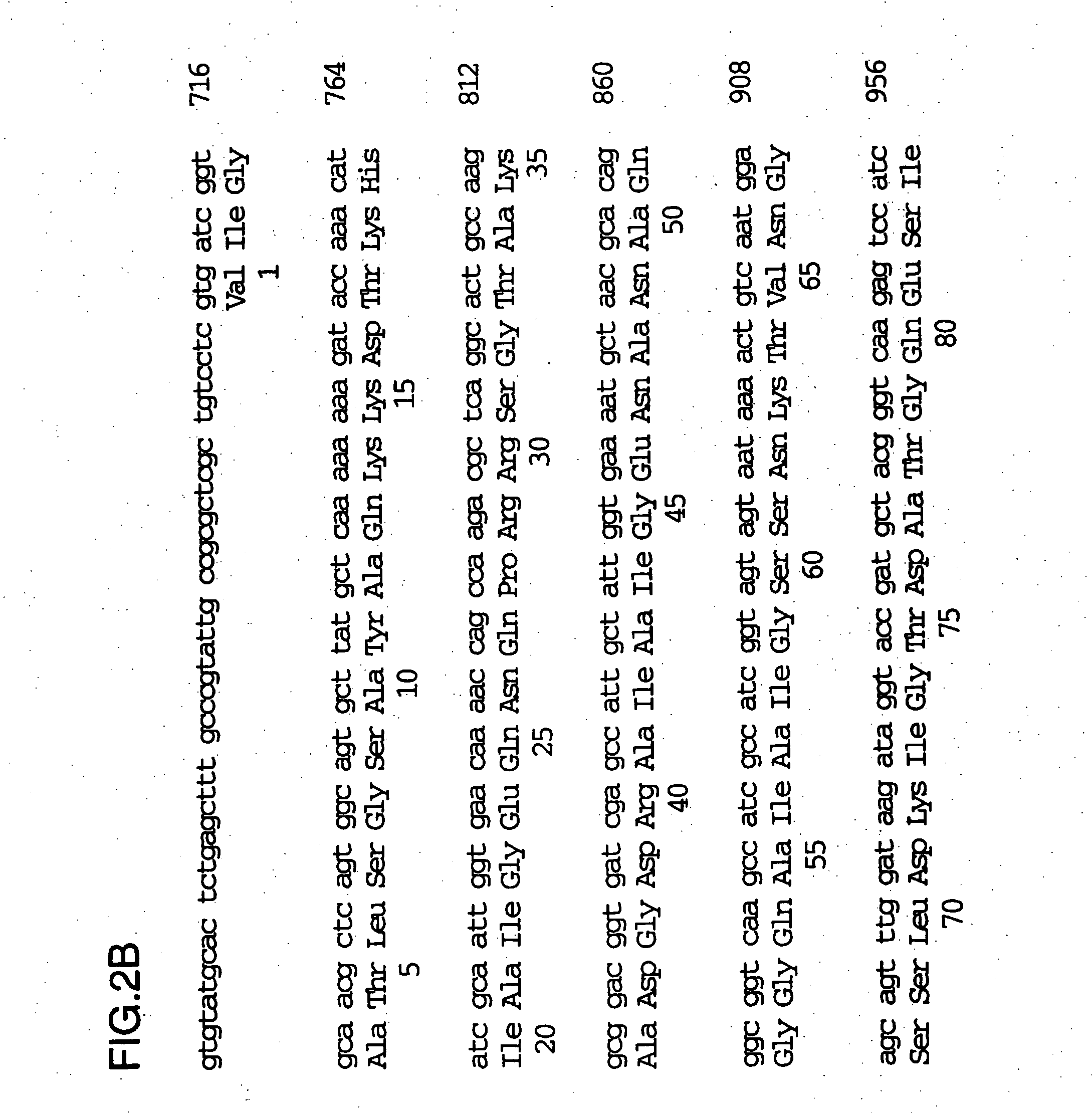
An isolated and purified outer membrane protein of a Moraxella strain, particularly M. catarrhalis, has a molecular mass of about 200 kDa. The about 200 kDa outer membrane protein as well as nucleic acid molecules encoding the same are useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a bacterial pathogen that produces the about 200 kDa outer membrane protein or produces a protein capable of inducing antibodies in a host specifically reactive with the about 200 kDa outer membrane protein.
Owner:AVENTIS PASTUER LTD
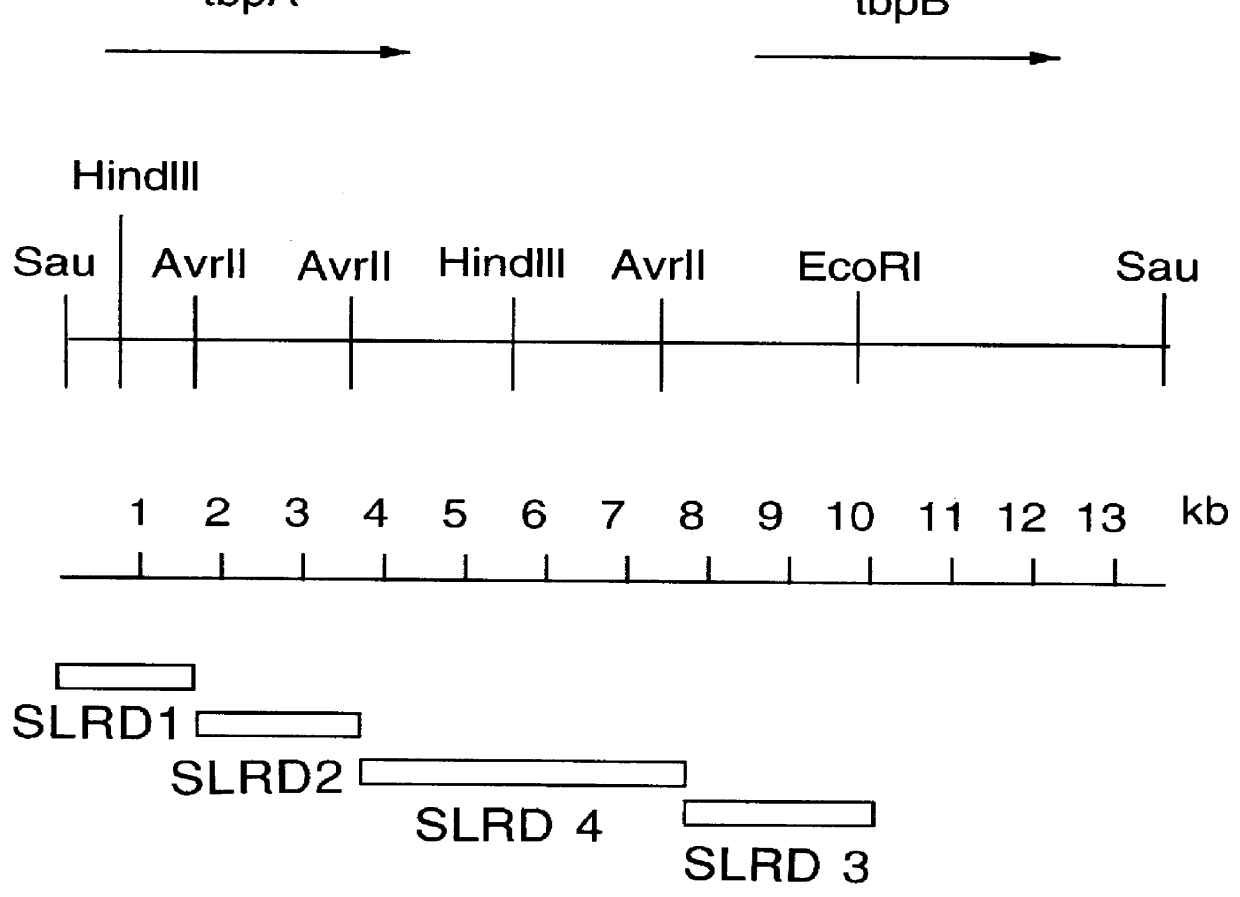
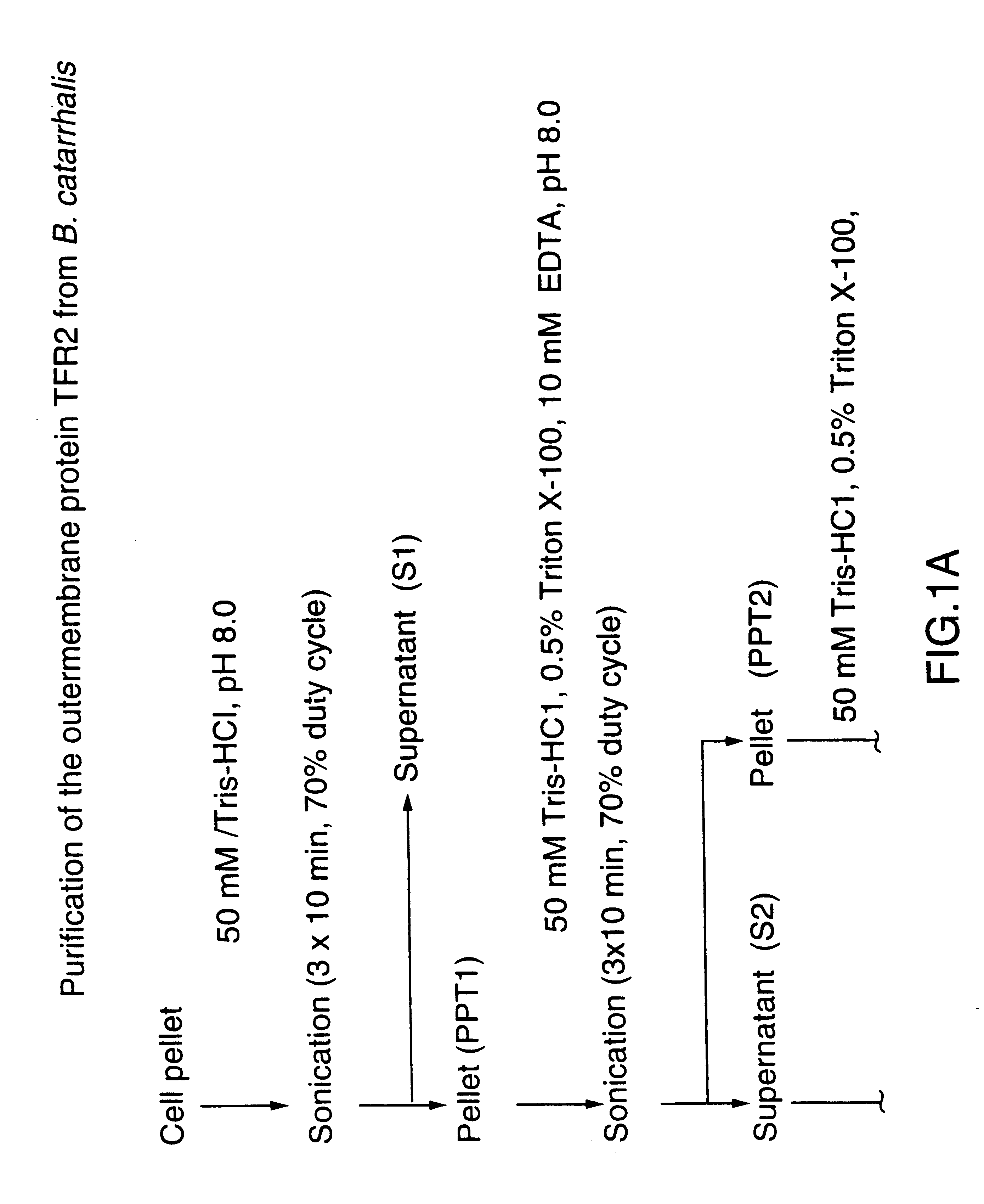
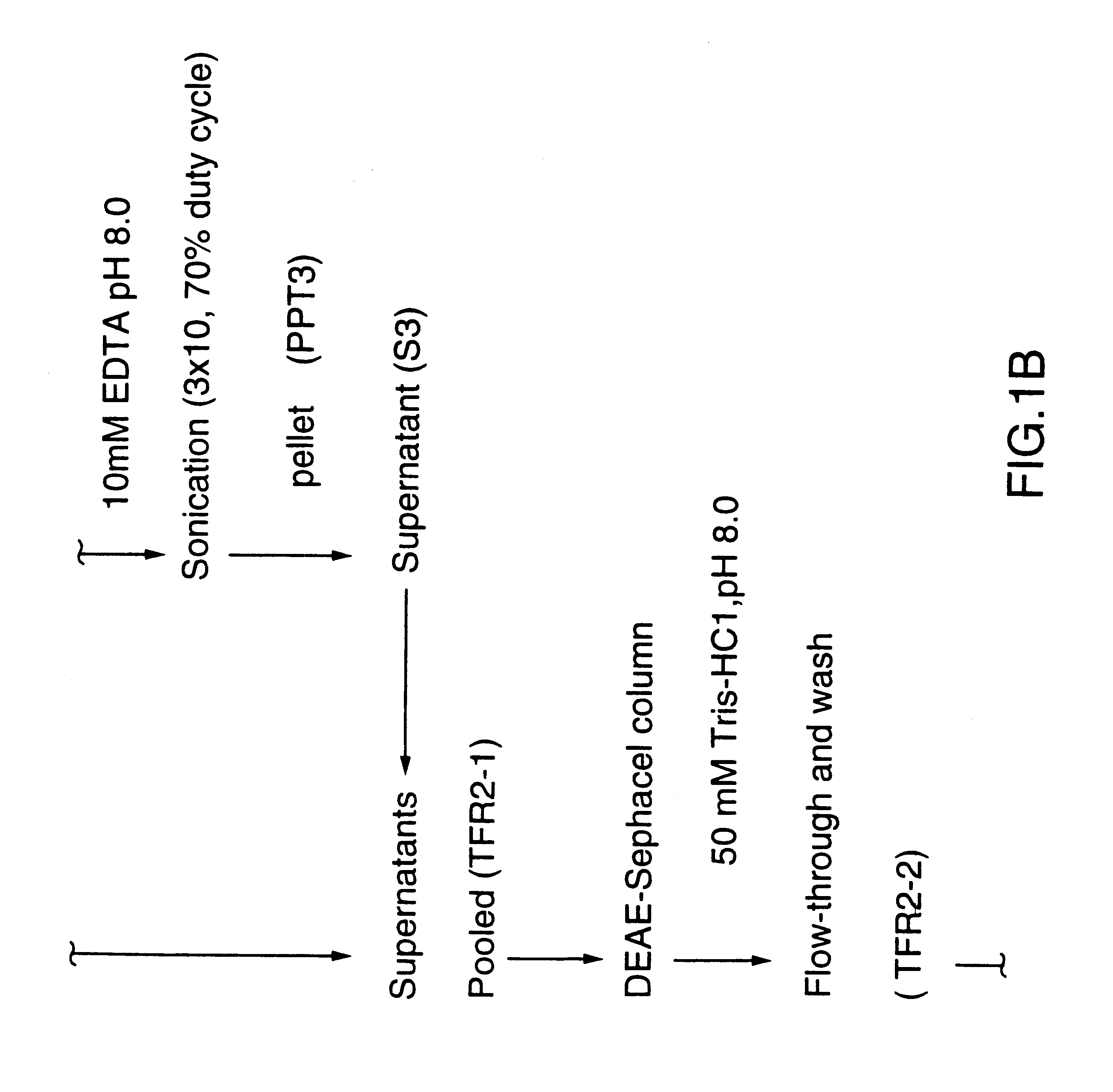
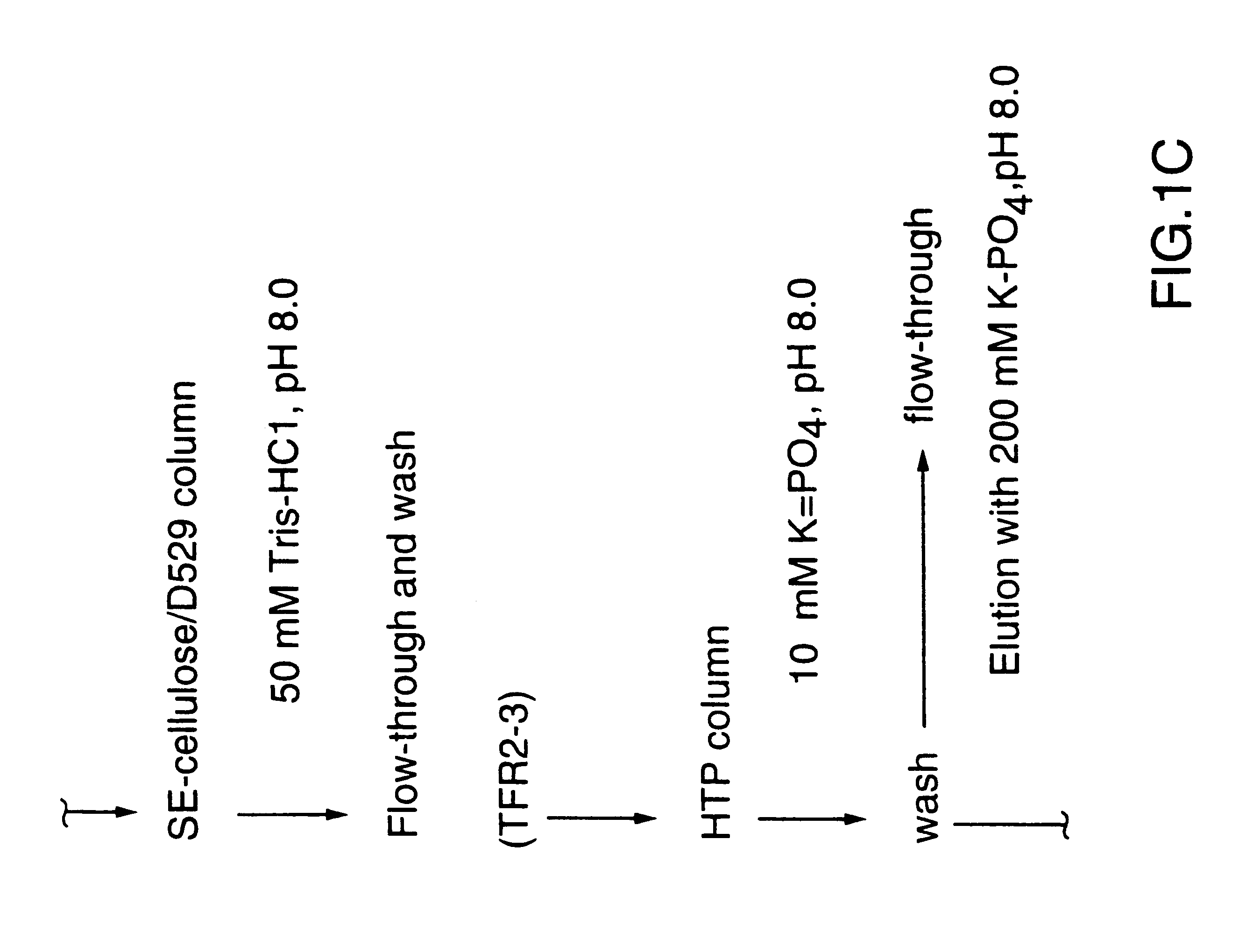
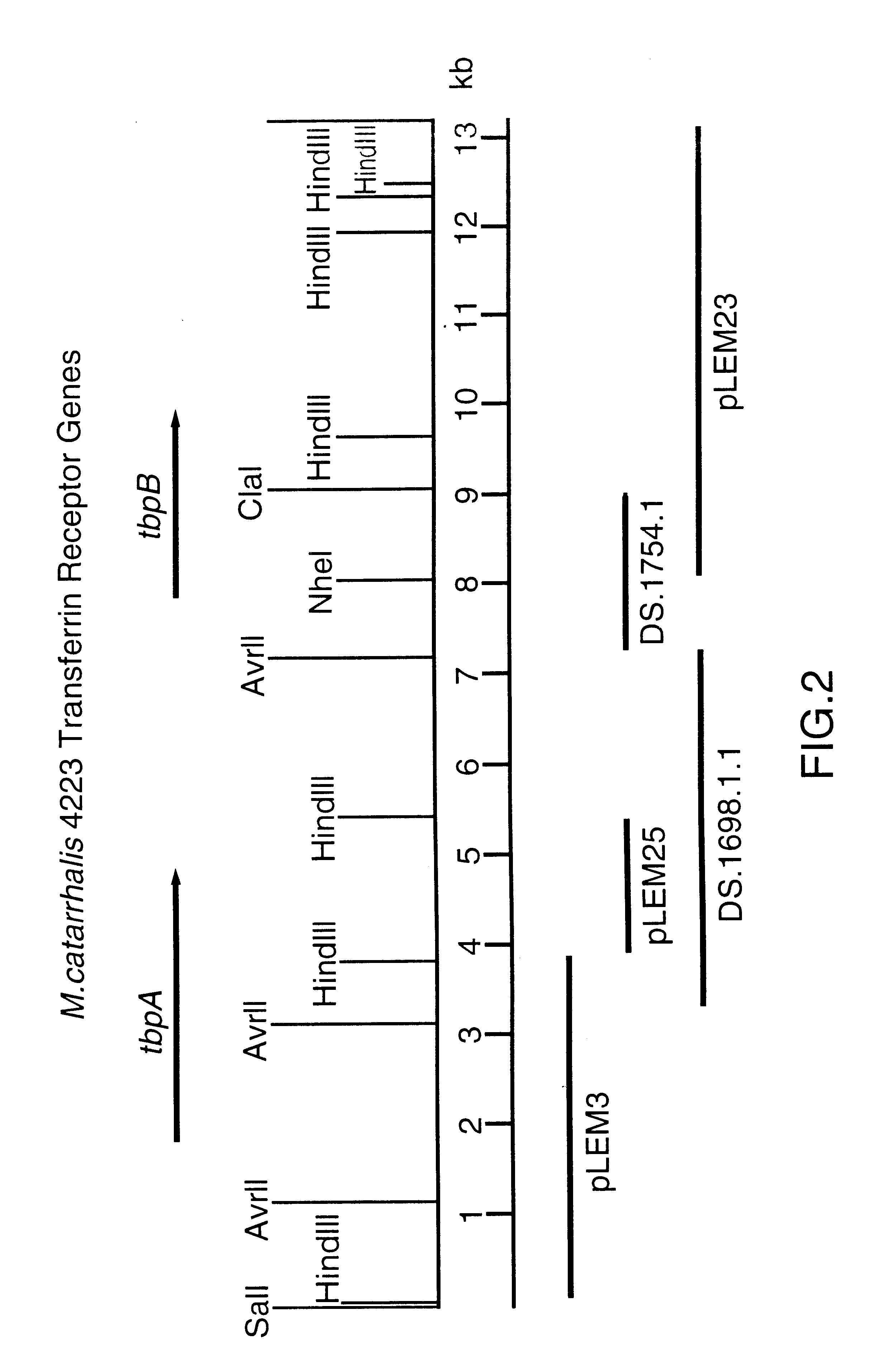
DNA encoding a transferrin receptor of Moraxella
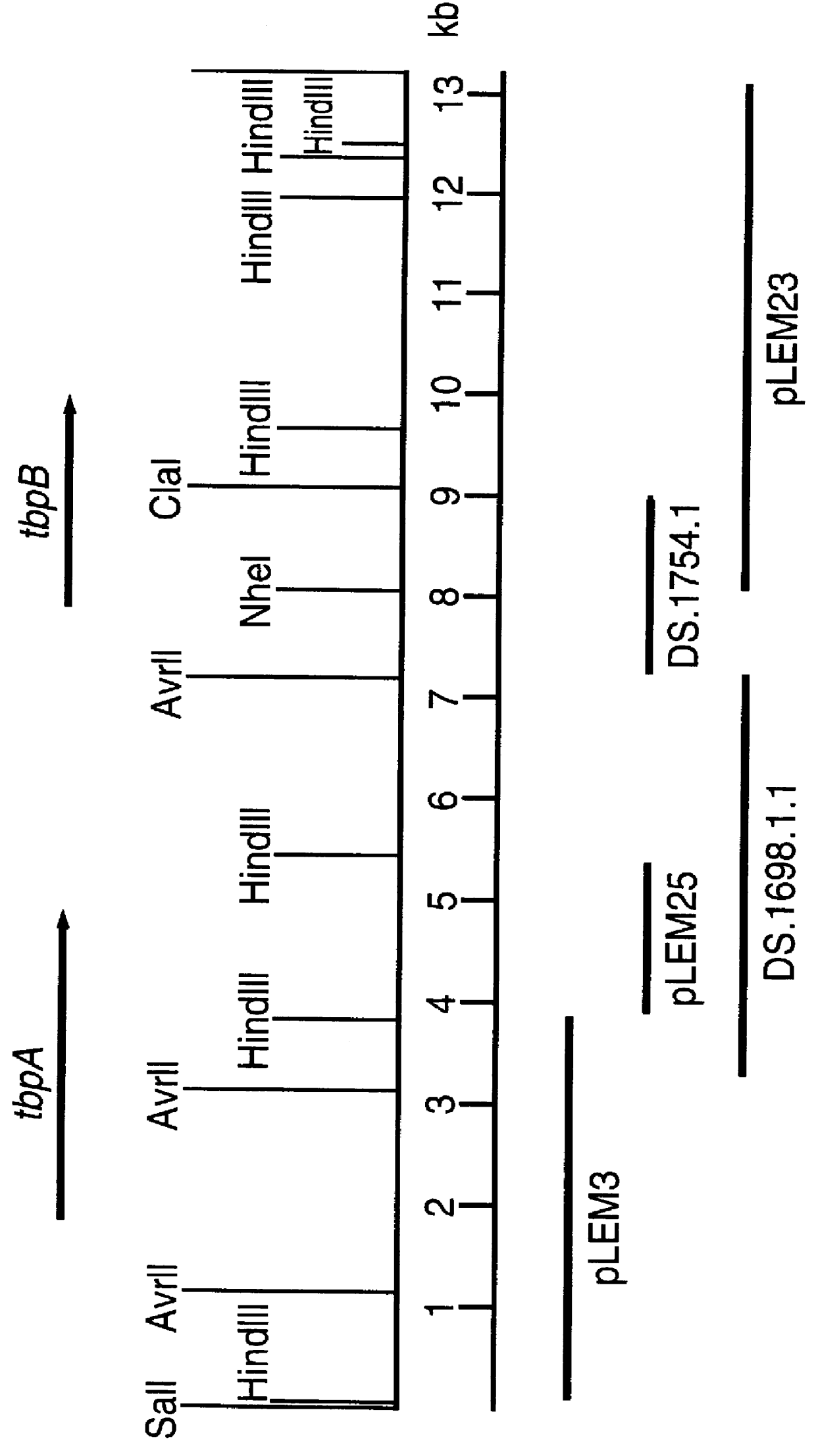
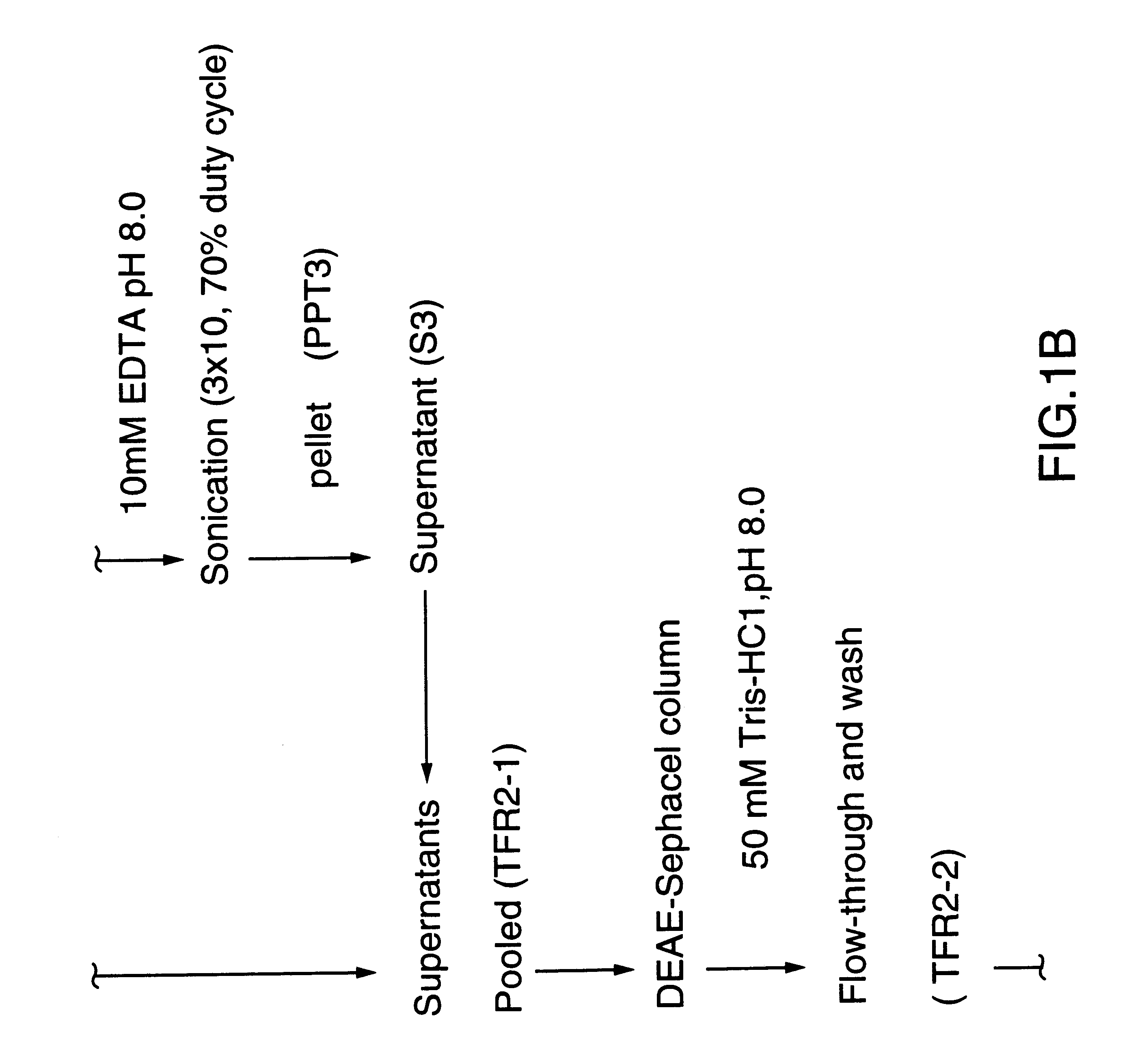
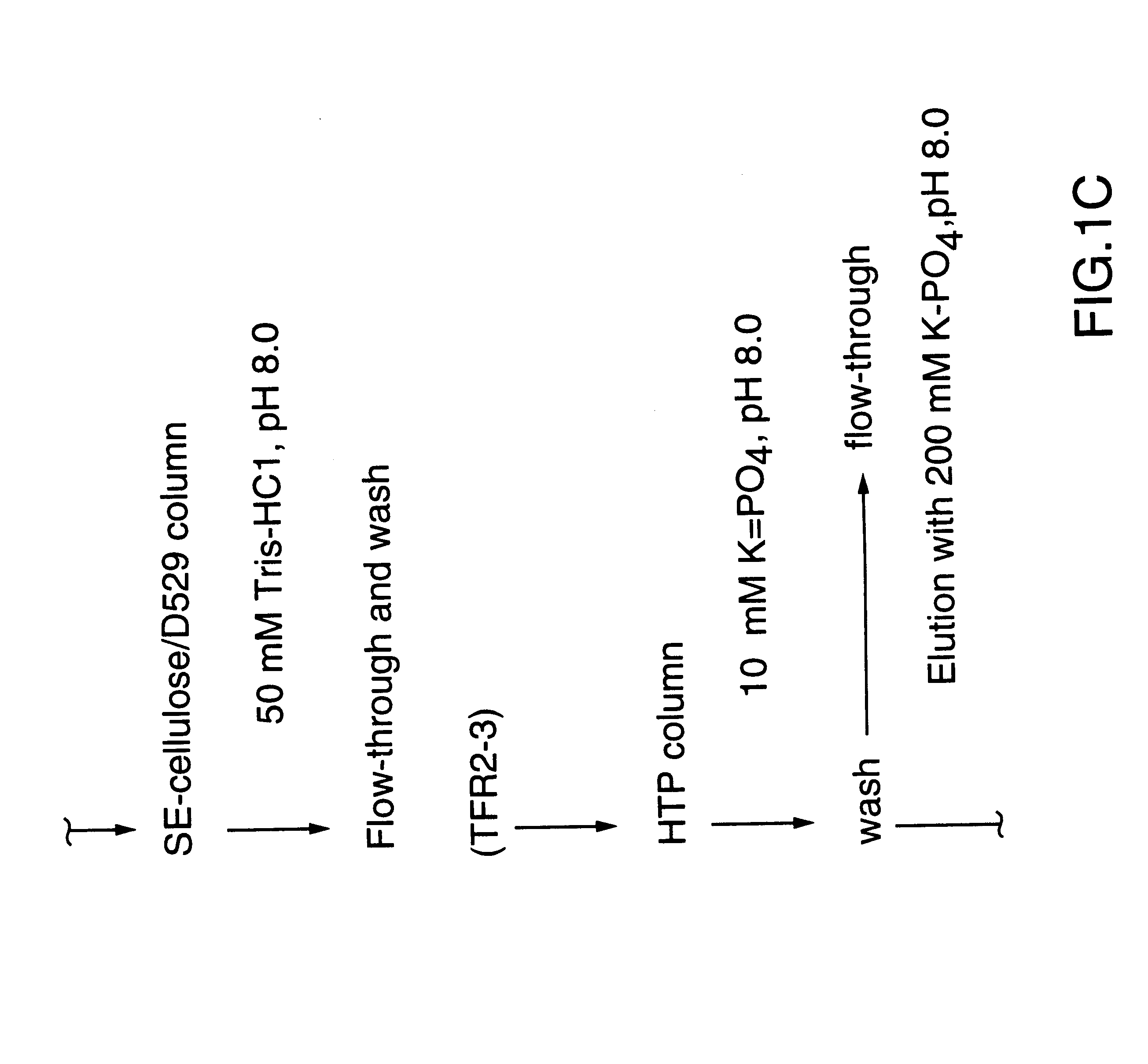
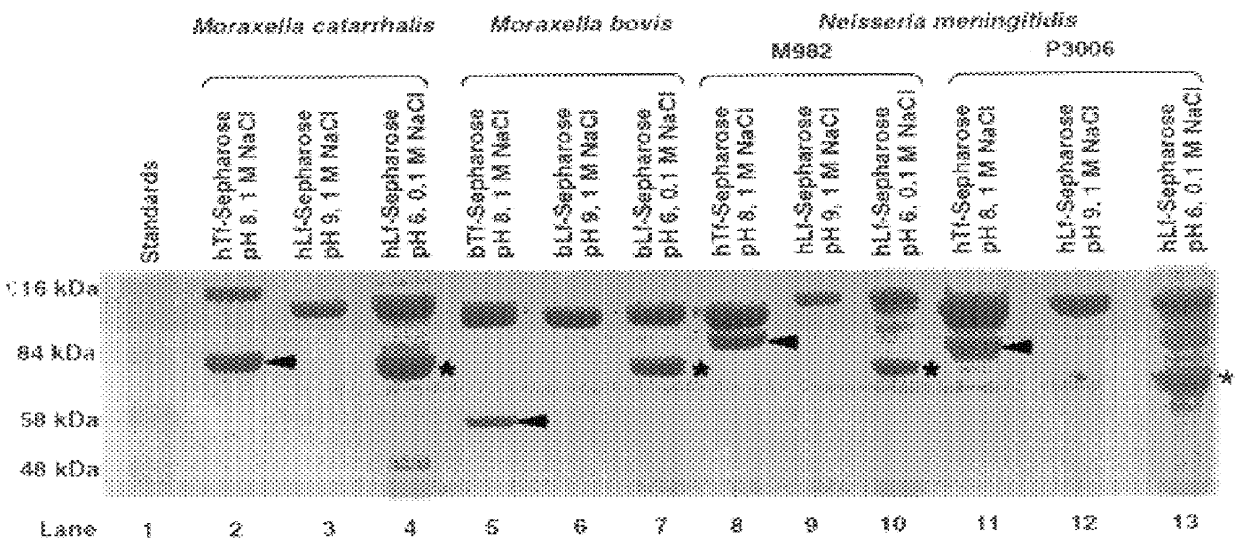
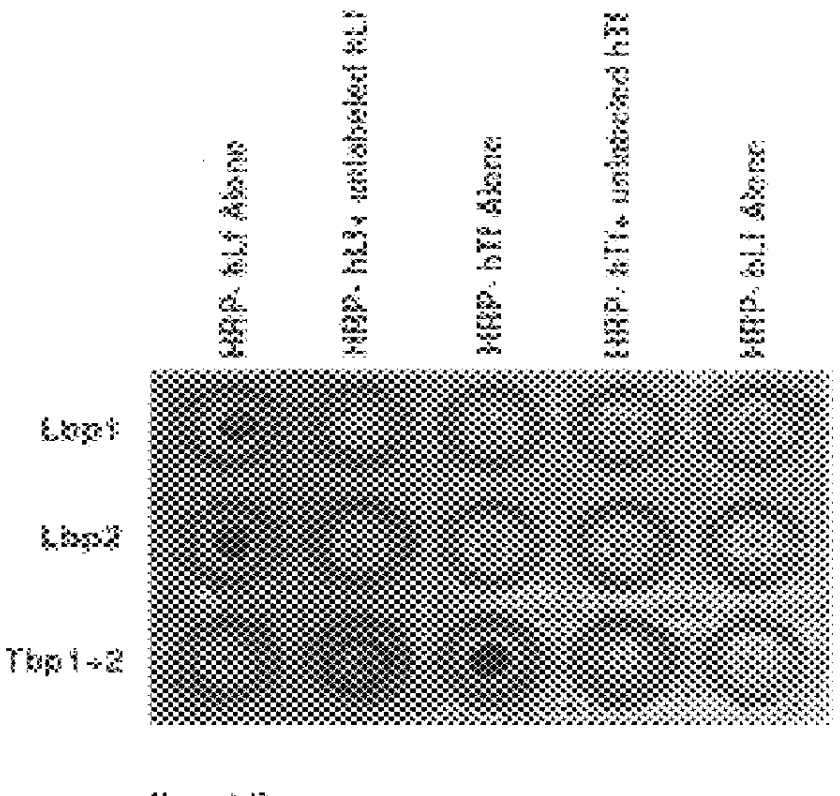
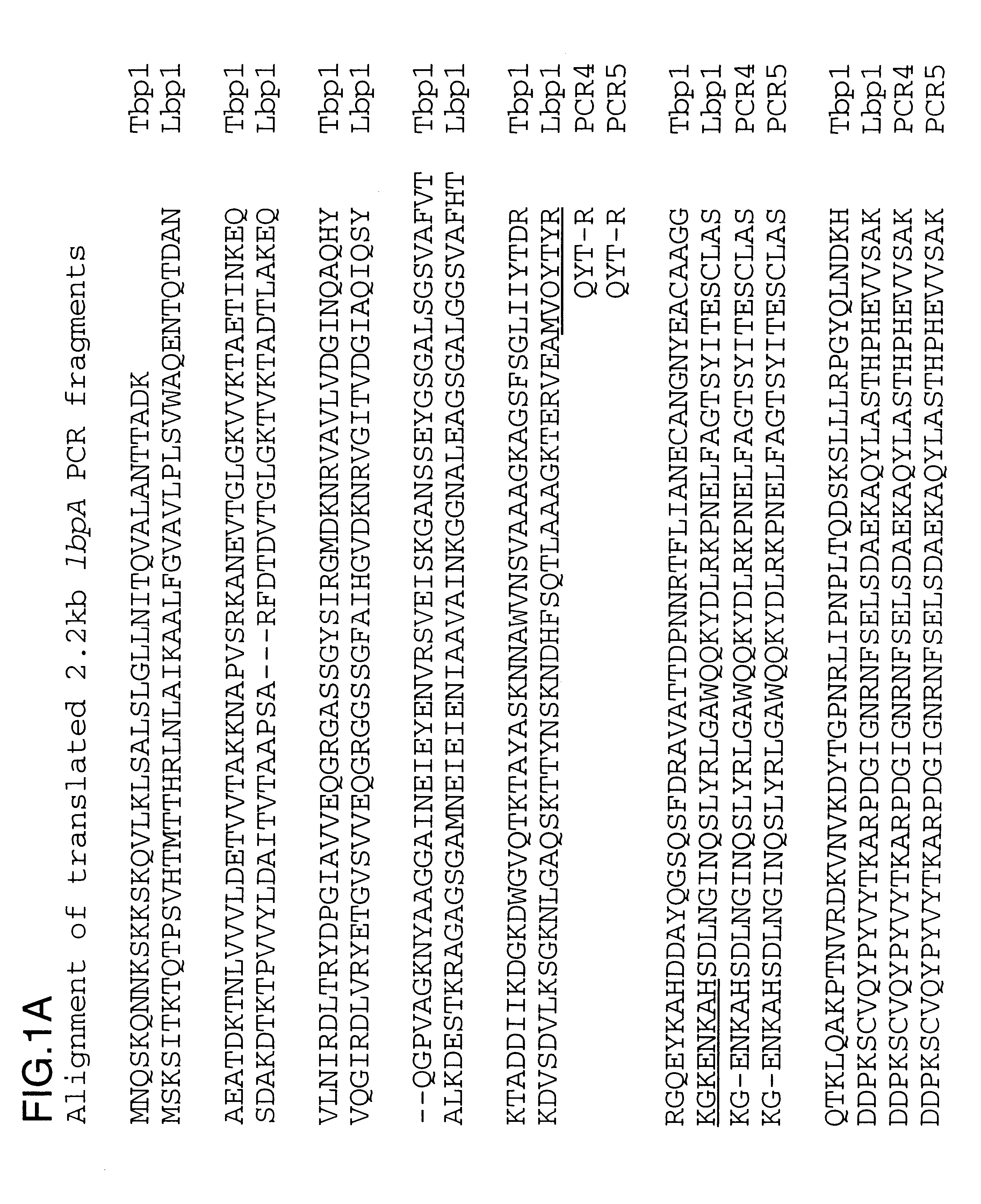
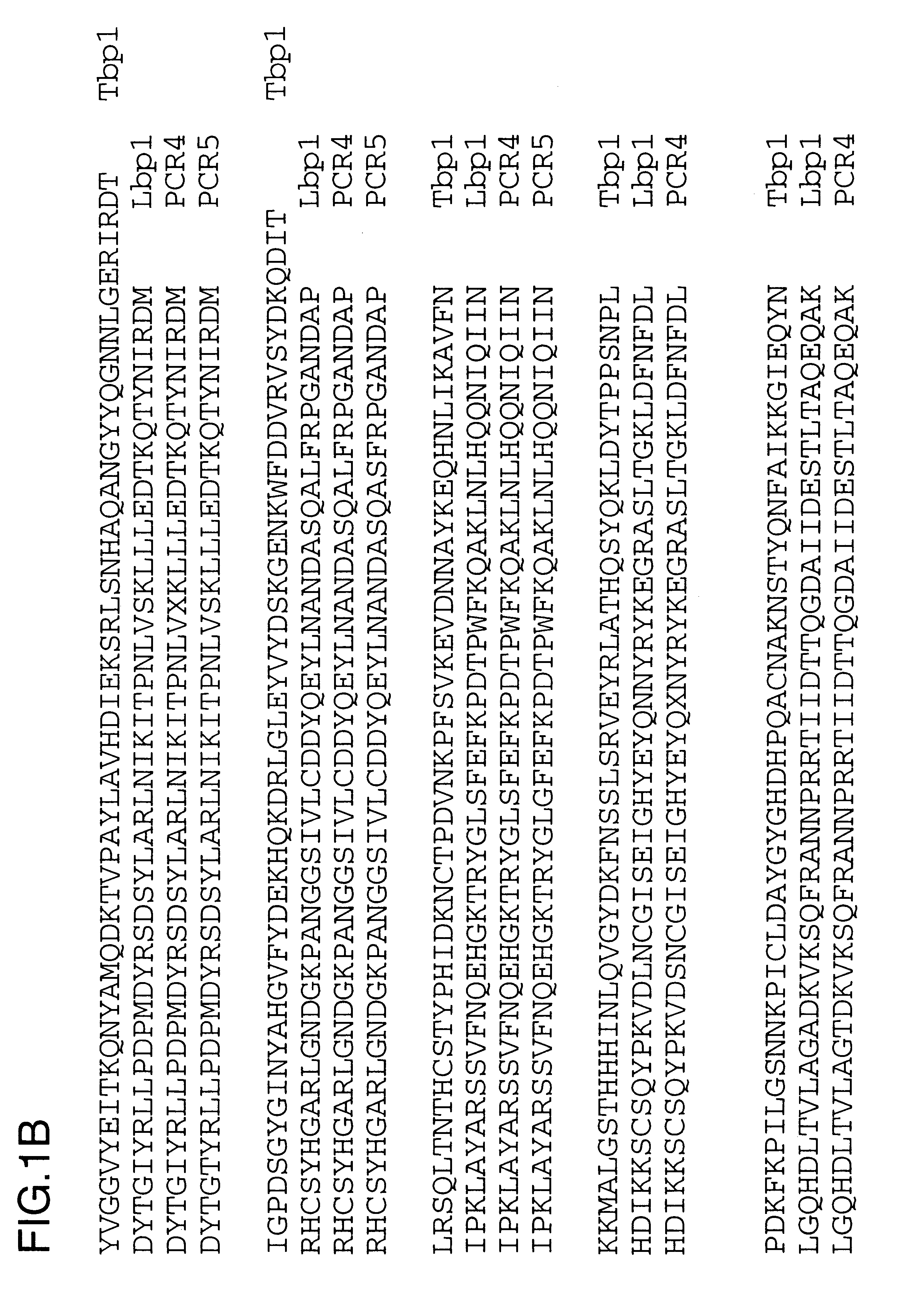
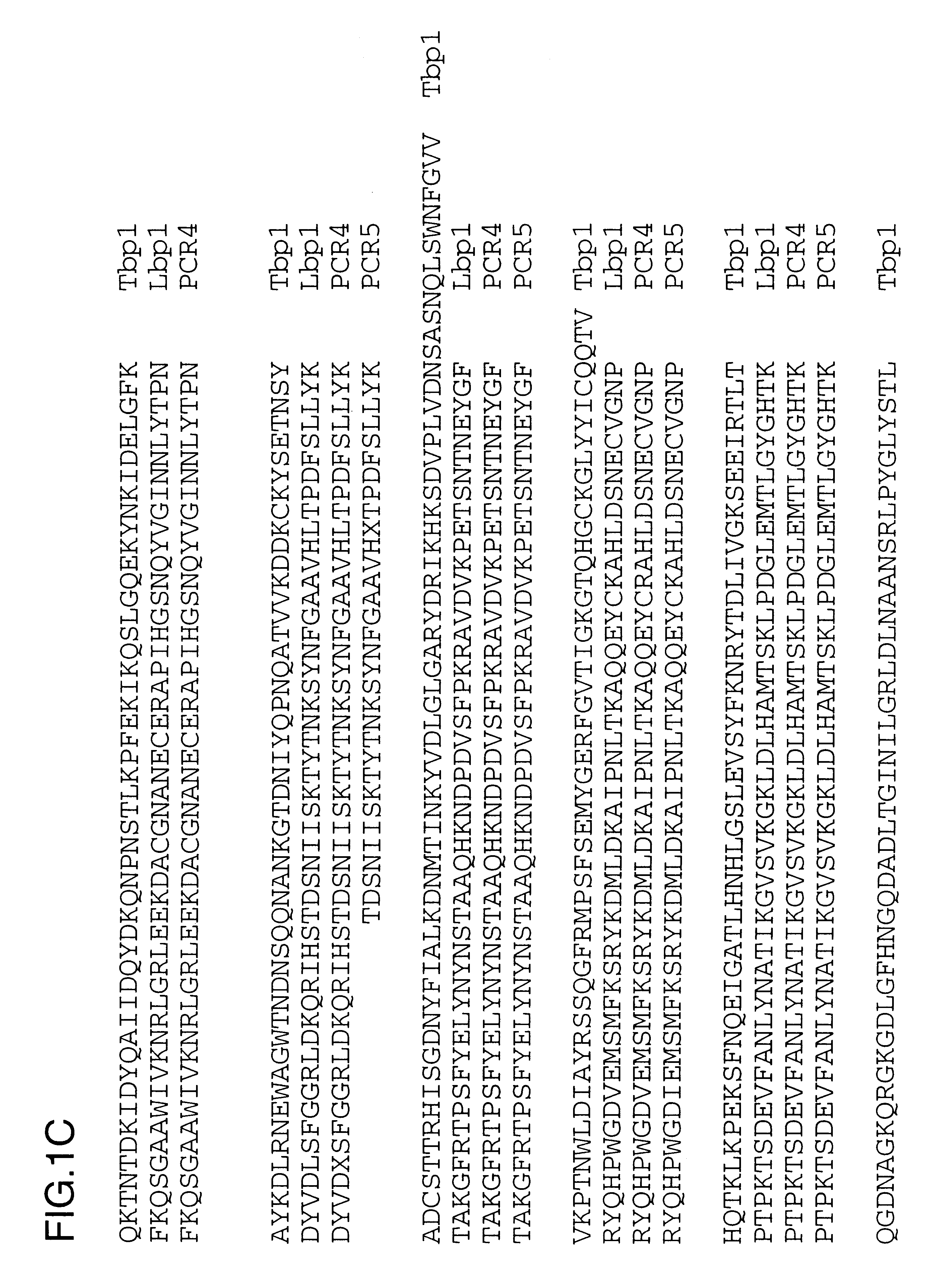
Purified and isolated nucleic acid molecules are provided which encode transferrin receptor proteins of Moraxella, such as M. catarrhalis or a fragment or an analog of the transferrin receptor protein. The nucleic acid sequence may be used to produce recombinant transferrin receptor proteins Tbp1 and Tbp2 of the strain of Moraxella free of other proteins of the Moraxella strain for purposes of diagnostics and medical treatment. Furthermore, the nucleic acid molecule may be used in the diagnosis of infection.
Owner:CONNAUGHT LAB
Nucleic acids encoding 3-ketoacyl-ACP reductase from Moraxella catarrahalis
InactiveUS6632636B1Extended half-lifeIncrease intracellular stabilitySugar derivativesBacteriaPDAT enzymeOpen reading frame
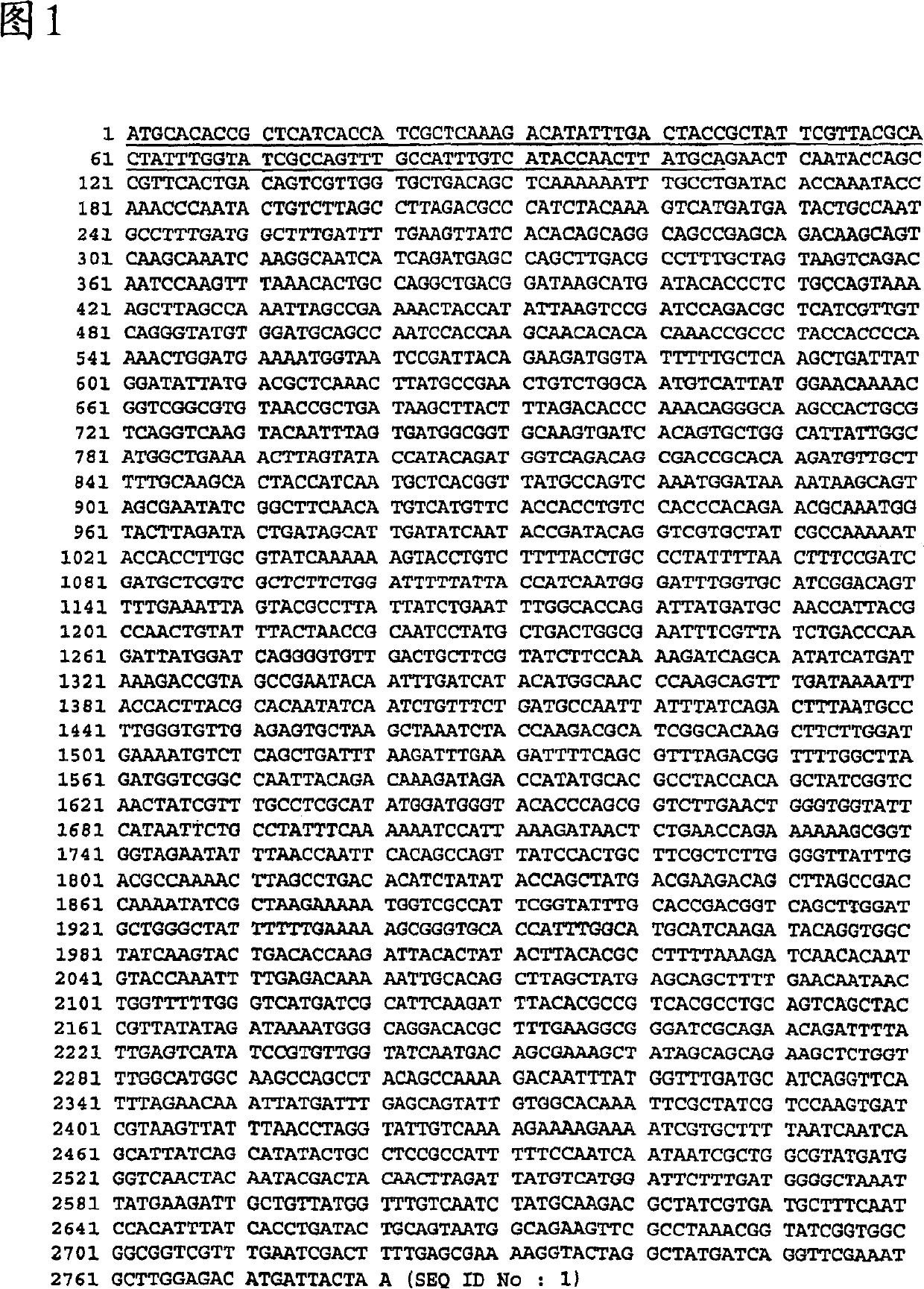
The present invention provides the genomic sequences of a library of purified nucleic acid molecules, or their complements, comprising the genome of <HIL><PDAT>Moraxella catarrhalis< / ITALIC><PDAT>. The invention also provides the identification of open reading frames contained within the nucleic acid molecules of the library. The present invention further provides for the use of the nucleic acid molecules, their complements or fragments, and proteins or portions thereof for identifying ligands and useful diagnostic and therapeutic compositions. In addition the invention provides for vectors, host cells and methods for producing <HIL><PDAT>M. catarrhalis < / ITALIC><PDAT>proteins or portions thereof.< / PTEXT>
Owner:MERCK & CO INC
High molecular weight major outer membrane protein of moraxella
InactiveUS6440424B1Improving immunogenicitySlow and sustained releaseBiocidePeptide/protein ingredientsDiseaseIn vivo
Owner:AVENTIS PASTEUR LTD
High molecular weight major outer membrane protein of moraxella
InactiveUS20020068070A1Improving immunogenicitySlow and sustained releaseAntibacterial agentsVirusesDiseaseIn vivo
An isolated and purified outer membrane protein of a Moraxella strain, particularly M. catarrhalis, having a molecular mass of about 200 kDa, is provided. The about 200 kDa outer membrane protein as well as nucleic acid molecules encoding the same are useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a bacterial pathogen that produces the about 200 kDa outer membrane protein or produces a protein capable of inducing antibodies in a host specifically reactive with the about 200 kDa outer membrane protein.
Owner:AVENTIS PASTEUR LTD
Transferrin receptor protein of Moraxella
InactiveUS6290970B1Improving immunogenicityImprove responseAntibacterial agentsBiocideDiseaseIn vivo
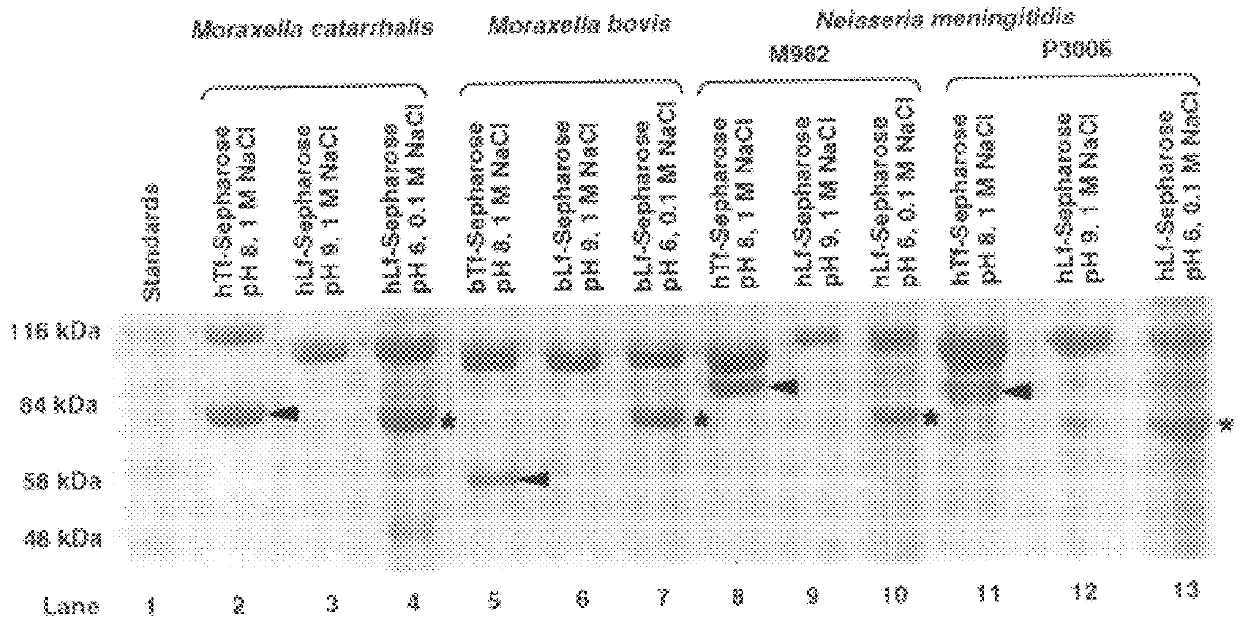
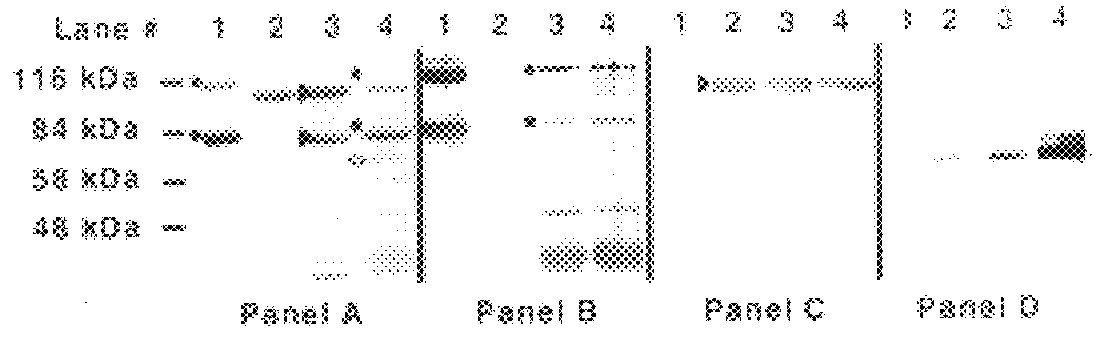
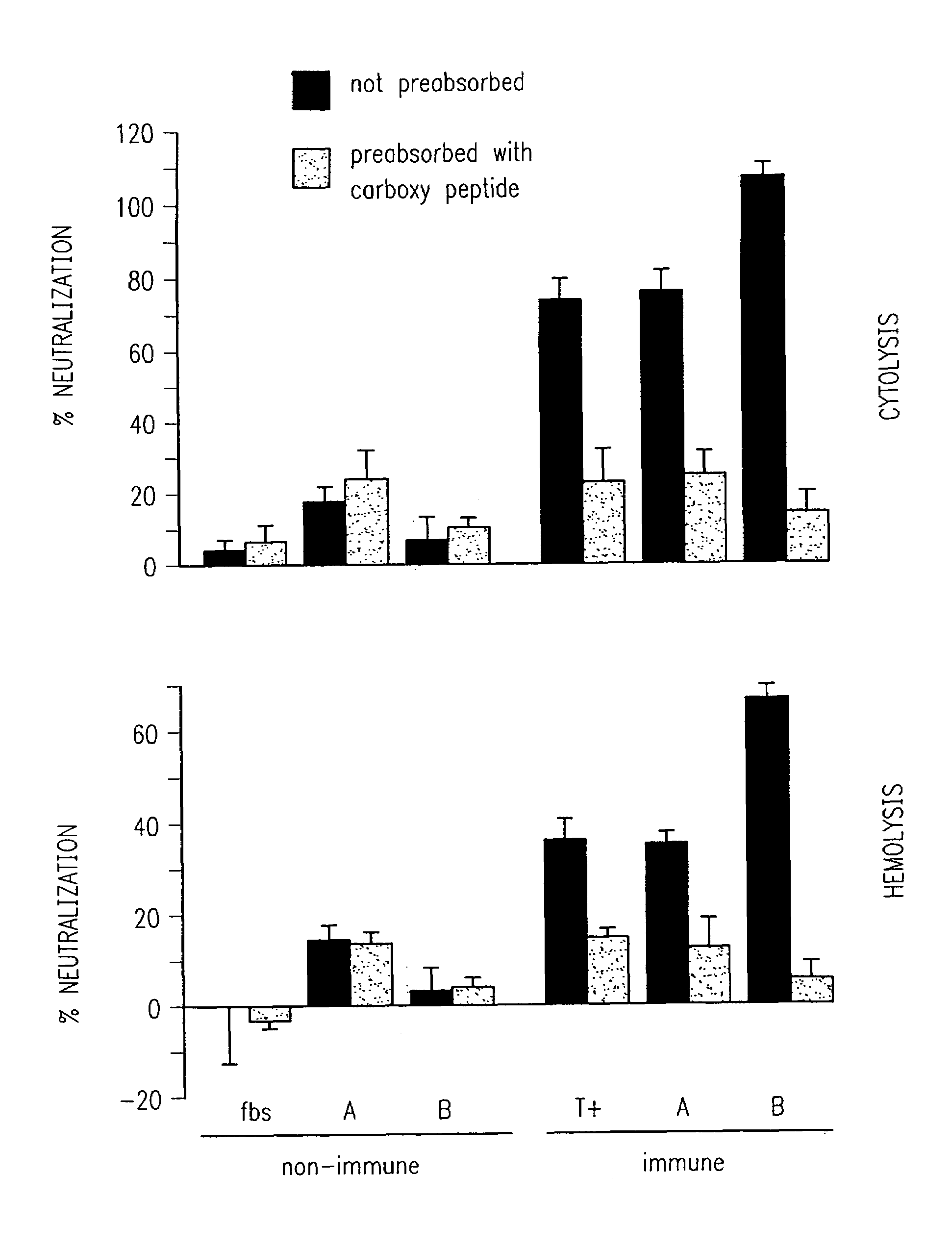
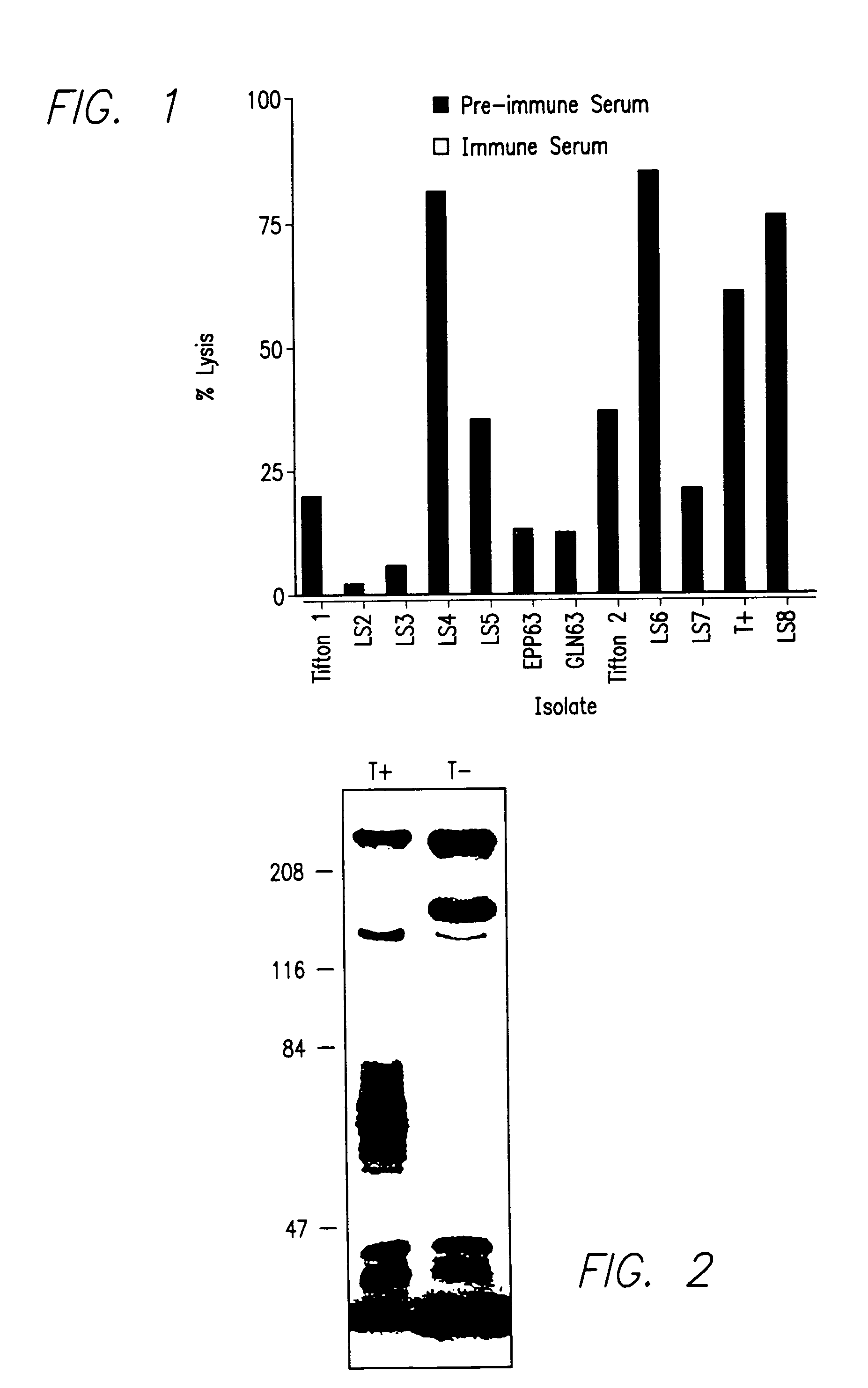
An isolated and purified non-denatured transferrin receptor protein of a Moraxella strain, particularly M. catarrhalis, has an apparent molecular mass of about 80 to about 90 kDa, as determined by SDS-PAGE. The transferrin receptor protein or a fragment analog thereof is useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a strain of Moraxella.
Owner:AVENTIS PASTEUR LTD
Transferrin receptor protein of moraxella
InactiveUS6190668B1Improving immunogenicityImprove responseAntibacterial agentsSenses disorderDiseaseCell mass
An isolated and purified non-denatured transferrin receptor protein of a Moraxella strain, particularly M. catarrhalis, has an apparent molecular mass of about 80 to about 90 kDa, as determined by SDS-PAGE. The transferrin receptor protein or a fragment analog thereof is useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a strain of Moraxella. The transferrin receptor protein is isolated from strains of Moraxella catarrhalis by a procedure including extraction of agent soluble proteins of a cell mass produced by cultivating the strain under iron-starved conditions. The transferrin receptor protein is selectively solubilized from the extracted cell mass and purified.
Owner:AVENTIS PASTUER LTD
Transferrin receptor of moraxella
Purified and isolated nucleic acid molecules are provided which encode transferrin receptor proteins of Moraxella, such as M. catarrhalis or a fragment or an analog of the transferrin receptor protein. The nucleic acid sequence may be used to produce recombinant transferrin receptor proteins Tbp1 and Tbp2 of the strain of Moraxella free of other proteins of the Moraxella strain for purposes of diagnostics and medical treatment. Furthermore, the nucleic acid molecule may be used in the diagnosis of infection.
Owner:AVENTIS PASTEUR LTD
Lactoferrin receptor protein
InactiveUS6211343B1Avoid accessImproving immunogenicityAntibacterial agentsDepsipeptidesBacteroidesDisease
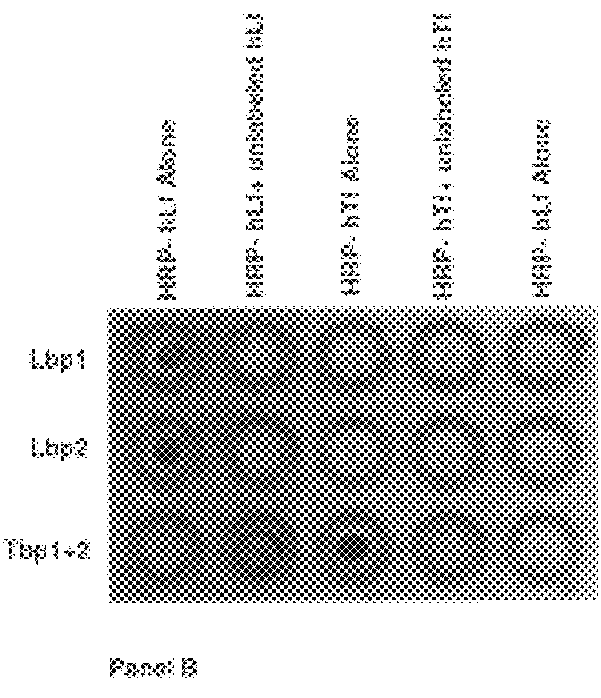
An isolated and purified lactoferrin receptor protein is isolated and purified from bacterial pathogens, including Moraxella and Neisseria, and has a molecular weight of between about 70,000 and about 90,000, as determined by SDS-PAGE. Such lactoferrin receptor protein may be provided in combination with a lactoferrin receptor protein from the bacterial pathogen of a molecular weight of about 100,000 to about 105,000 daltons. The lactoferrin receptor protein may be produced by providing a solubilized membrane preparation from the bacterial pathogen containing lactoferrin receptor proteins, non-lactoferrin receptor proteins and other contaminants, complexing the lactoferrin receptor proteins with lactoferrin and purifying the resulting complexes substantially free from the non-lactoferrin receptor proteins and the other contaminants, and separating the novel lactoferrin receptor protein from the complexes. The lactoferrin receptor protein is useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a bacterial pathogen that produces the lactoferrin receptor protein or produces a protein capable of inducing antibodies in a host specifically reactive with the lactoferrin receptor protein.
Owner:AVENTIS PASTEUR LTD
Lactoferrin receptor protein
InactiveUS6048539AAvoid accessProvide protectionAntibacterial agentsBacterial antigen ingredientsBacteroidesDisease
An isolated and purified lactoferrin receptor protein is isolated and purified from bacterial pathogens, including Moraxella and Neisseria, and has a molecular weight of between about 70,000 and about 90,000, as determined by SDS-PAGE. Such lactoferrin receptor protein may be provided in combination with a lactoferrin receptor protein from the bacterial pathogen of a molecular weight of about 100,000 to about 105,000 daltons. The lactoferrin receptor protein may be produced by providing a solubilized membrane preparation from the bacterial pathogen containing lactoferrin receptor proteins, non-lactoferrin receptor proteins and other contaminants, complexing the lactoferrin receptor proteins with lactoferrin and purifying the resulting complexes substantially free from the non-lactoferrin receptor proteins and the other contaminants, and separating the novel lactoferrin receptor protein from the complexes. The lactoferrin receptor protein is useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a bacterial pathogen that produces the lactoferrin receptor protein or produces a protein capable of inducing antibodies in a host specifically reactive with the lactoferrin receptor protein.
Owner:CONNAUGHT LAB
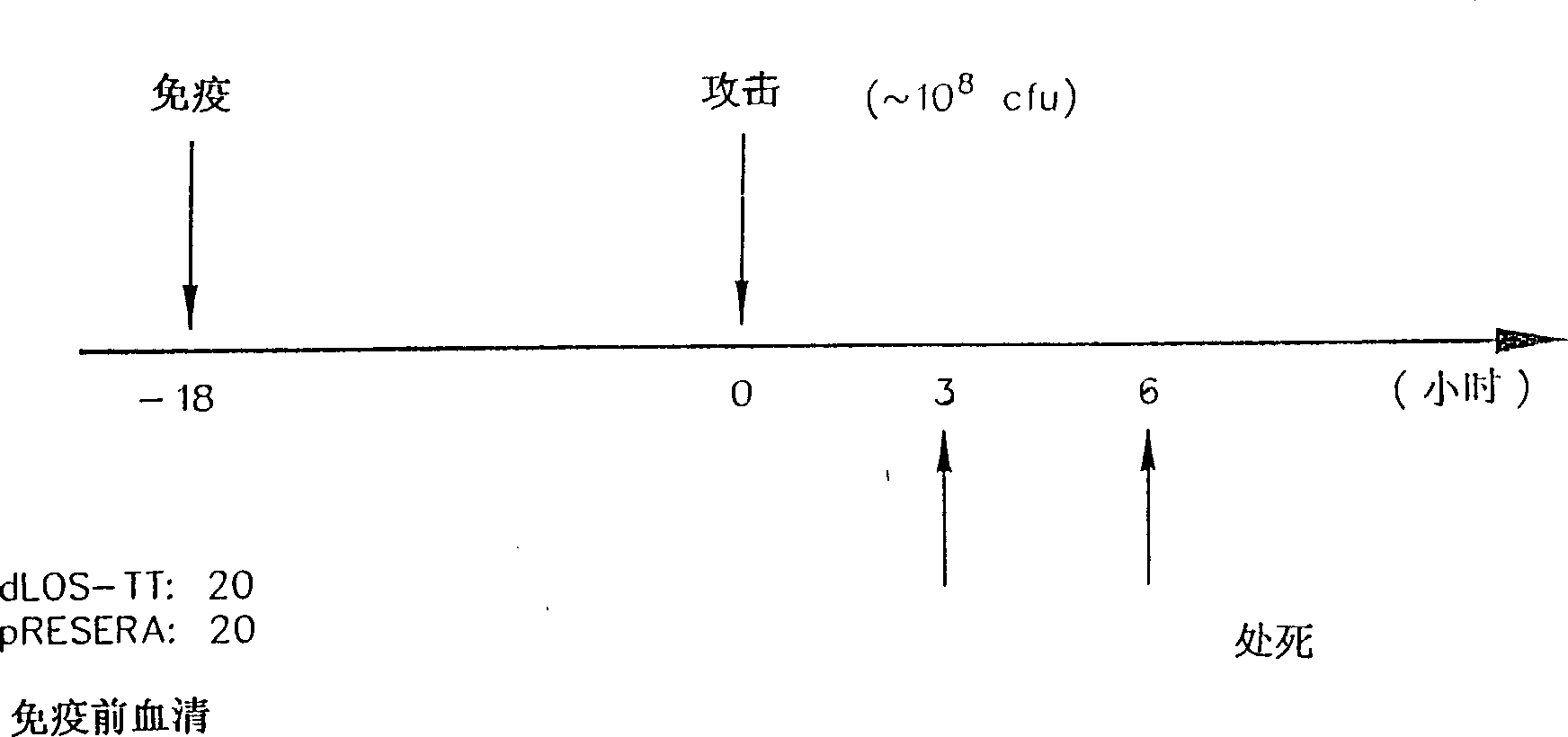
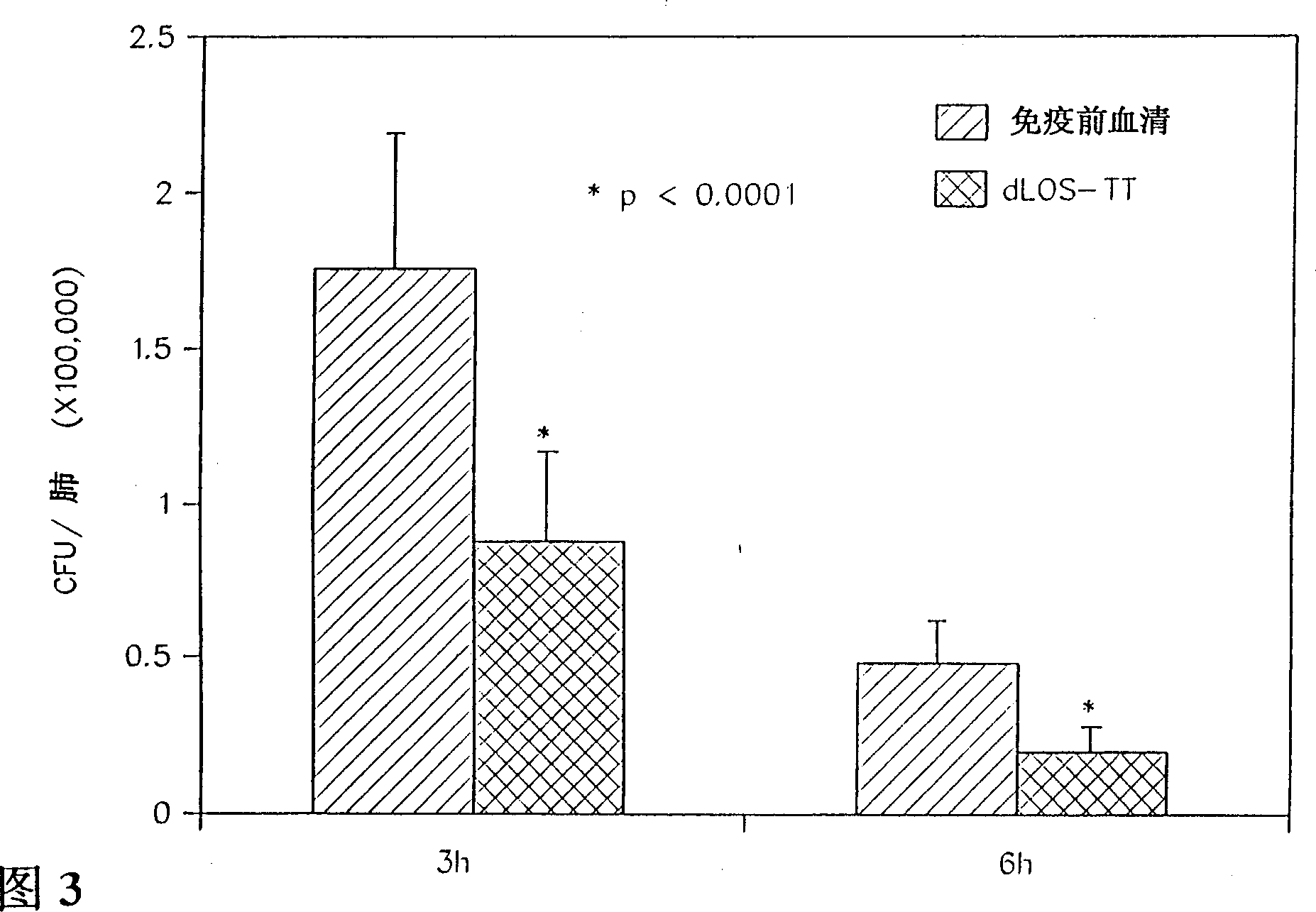
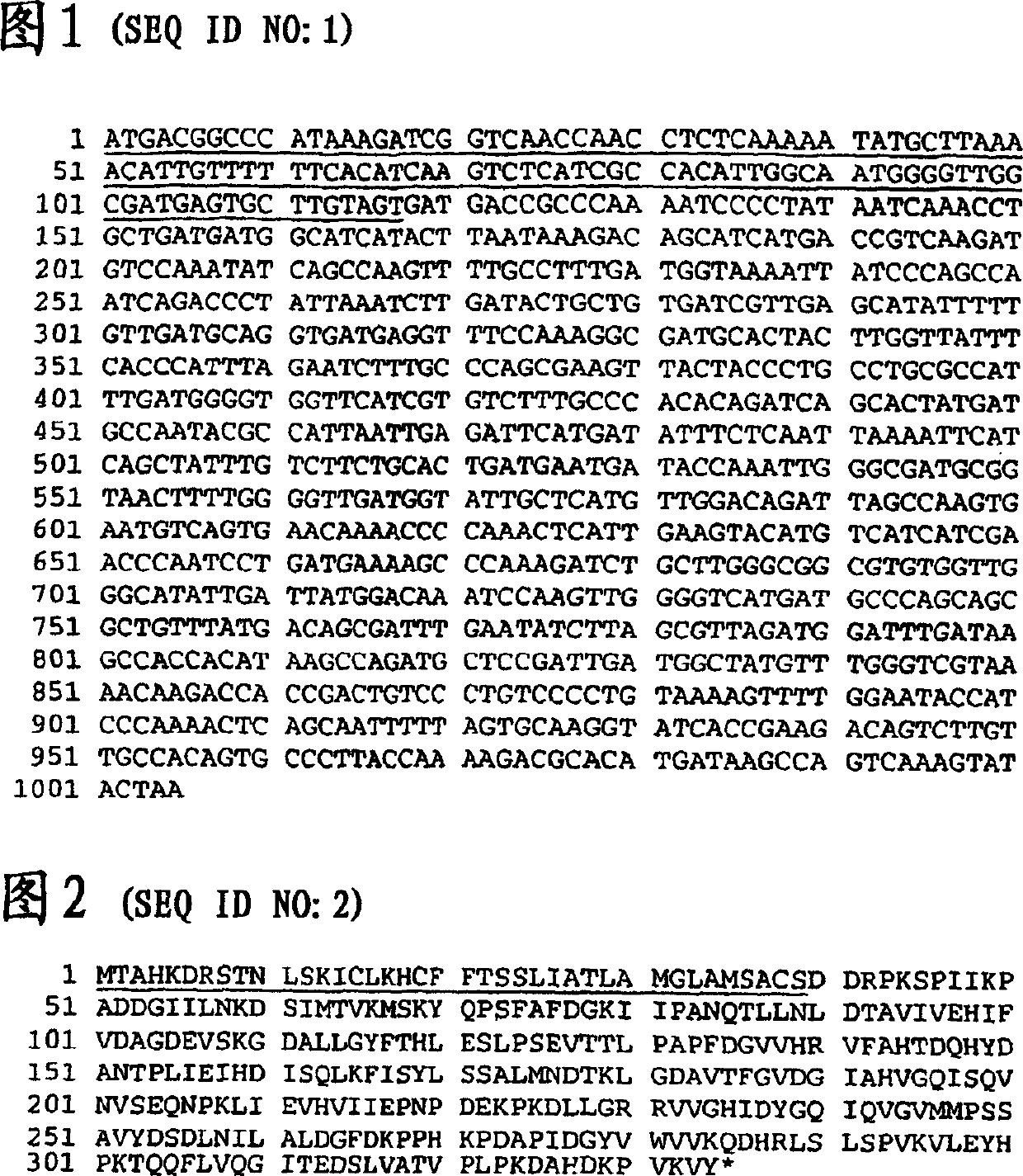
Lipooligosaccharide-based vaccine for prevention of i(moraxella) i(branhamella) i (catarrhalis) infections in mammals
A conjugate vaccine for Moraxella (Branhamella) catarrhalis comprising isolated lipooligosaccharide from which esterified fatty acids have been removed, to produce a detoxified lipooligosaccharide (dLOS), or from which lipid A has been removed, to produce a detoxified oligosaccharide (OS), which is linked to an immunogenic carrier. The vaccine is useful for preventing otitis media and respiratory infections caused by M. catarrhalis in mammals, including humans.
Owner:UNITED STATES OF AMERICA
Moraxella (branhamella) catarrhalis antigens
Owner:ID BIOMEDICAL
Moraxella bovis cytotoxin, cytotoxin gene, antibodies and vaccines for prevention and treatment of Moraxella bovis infections
InactiveUS7220421B2Bacterial antigen ingredientsPeptide/protein ingredientsInfectious bovine keratoconjunctivitisNucleotide sequencing
Moraxella bovis cytotoxin and a gene encoding Moraxella bovis cytotoxin. Identification, isolation, cloning and identification of nucleotide sequence of the Moraxella bovis genes mbxA, mbxB, mbxC and mbxD, partial purification of the native cytotoxin, preparation of partially purified native and a recombinant Moraxella bovis cytotoxin, identification of an amino acid sequence of the cytotoxin, preparation of antibodies against the Moraxella bovis cytotoxin, preparation of vaccines against Moraxella bovis. Method for prevention and treatment of infectious bovine keratoconjunctivitis caused by Moraxella bovis.
Owner:RGT UNIV OF CALIFORNIA
Lactoferrin receptor genes of Moraxella
InactiveUS6184371B1Provide protectionImproving immunogenicityOrganic active ingredientsSenses disorderENCODENucleic acid sequencing
Purified and isolated nucleic acid molecules are provided which encode lactoferrin receptor proteins of Moraxella, such as M. catarrhalis, or a fragment or an analog of the lactoferrin receptor protein. The nucleic acid sequence may be used to produce recombinant lactoferrin receptor proteins Lbp1, Lbp2 and ORF3 of the strain of Moraxella free of other proteins of the Moraxella strain for purposes of diagnostics and medical treatment. Furthermore, the nucleic acid molecule may be used in the diagnosis of infection.
Owner:AVENTIS PASTUER LTD
Vaccine antigens of Moraxella
Owner:FARN JACINTA +2
Polypeptides of Moraxella (Branhamella) catarrhalis
Owner:ID BIOMEDICAL
Vaccine antigens of Moraxella
The present invention relates to antigens of Moraxella, in particular, Moraxella bovis, nucleic acid sequences encoding these antigens and formulations for use in raising an immune response against Moraxella.
Owner:COMMONWEALTH SCI & IND RES ORG +1
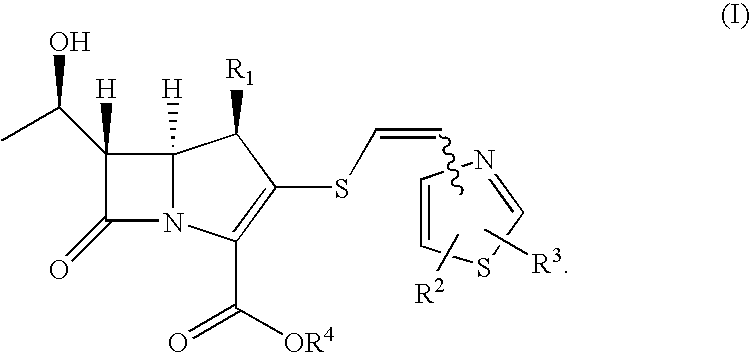
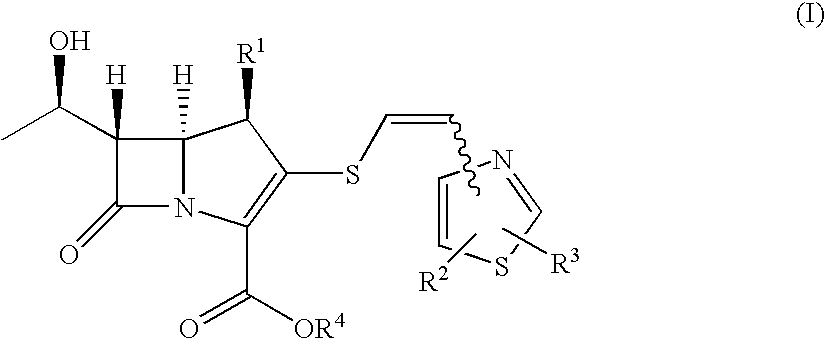
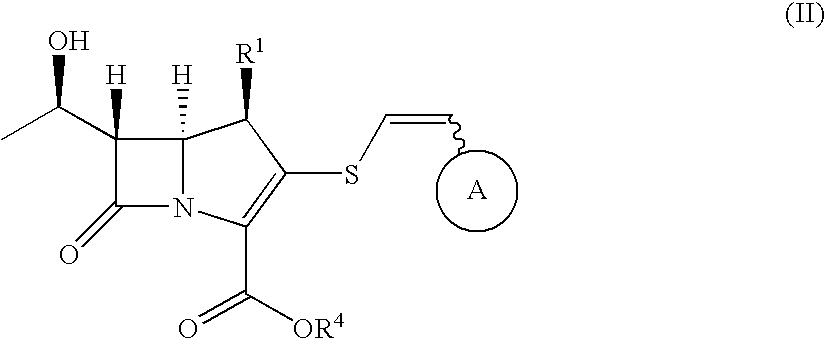
2-thioethenyl substituted carbapenem derivatives
InactiveUS7687490B2Broad spectrum of and potent anitimicrobial activityHigh antibacterial activityAntibacterial agentsSilicon organic compoundsResistant bacteriaPyrococcus
Owner:MEIJI SEIKA KAISHA LTD
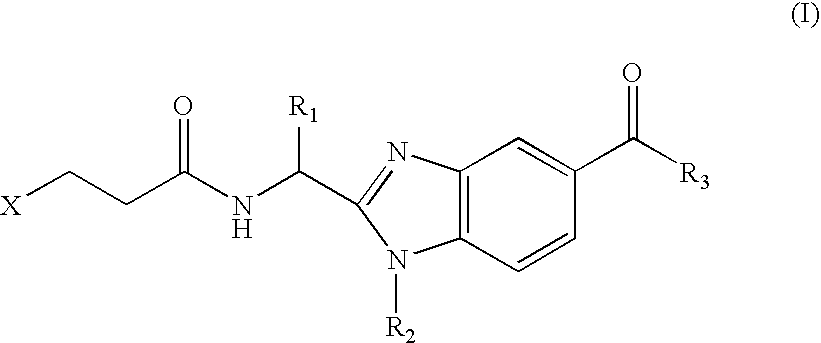
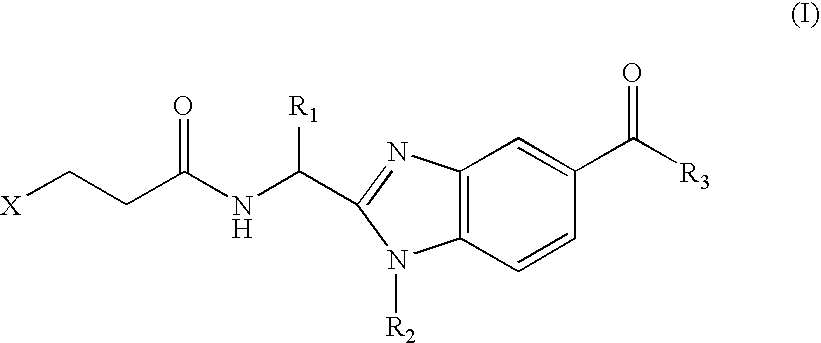
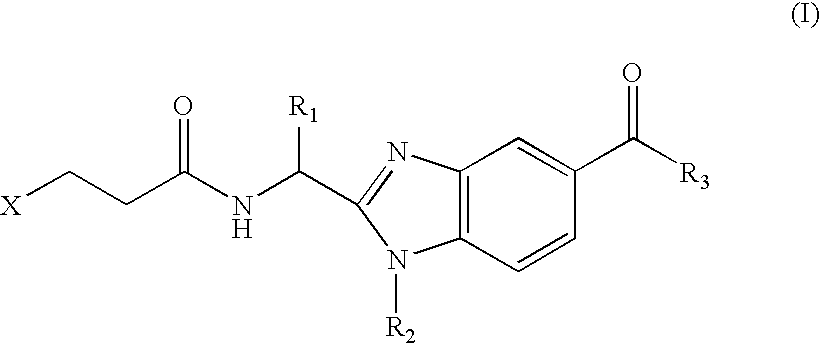
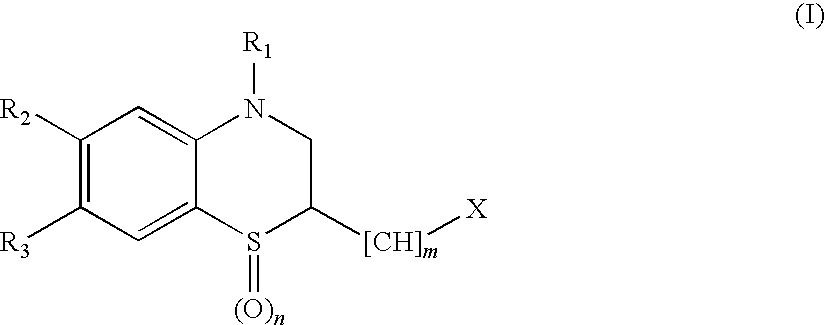
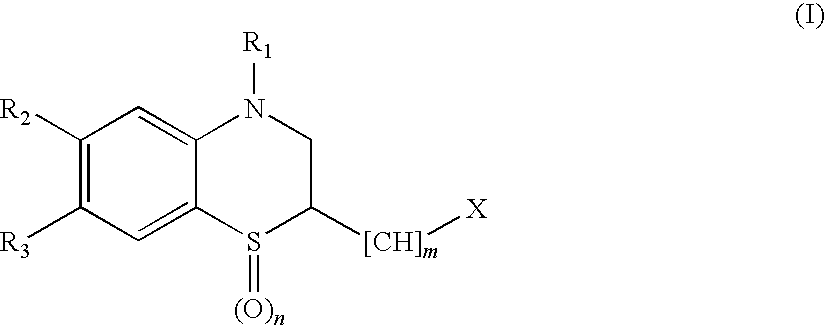
Benzimidazole derivatives and use thereof as peptide deformylase inhibitors
Benzimidazole compounds of the general formula (I) and pharmaceutically acceptable salts or esters thereof are peptide deformylase inhibitors useful in the treatment or prevention of infections and other diseases in which peptide deformylases are involved, especially in the treatment of bacterial and parasitic infections, for example infections fully or partly caused by microorganisms belonging to Staphylococcus, Enterococcus, Streptococcus, Haemophilus, Moraxella, Escherichia, Mycobacterium, Mycoplasma, Pseudomonas, Chlamydia, Rickettsia, Klebsiella, Shigella, Salmonella, Bordetella, Clostridium, helicobacter, Campylobacter, Legionella, or Neisseria.
Owner:ARPIDA AG
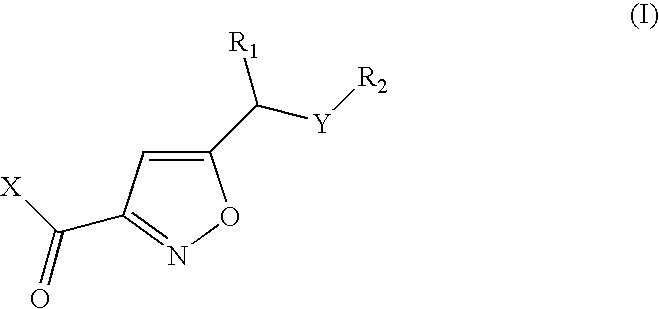
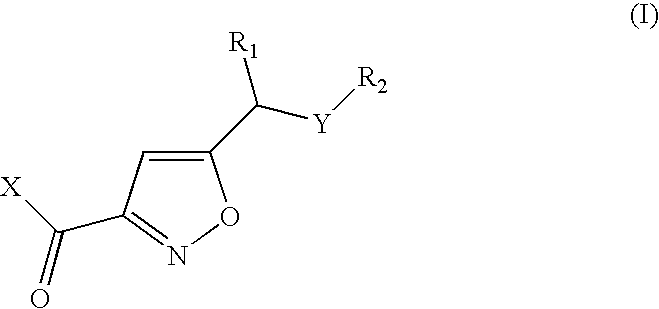
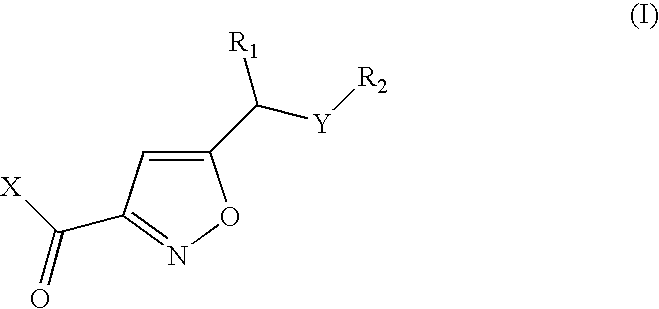
Isoxazoles as peptide deformylase inhibitors
Isoxazole compounds of formula (I) and pharmaceutically acceptable salts or esters thereof are peptide deformylase inhibitors useful in the treatment or prevention of infections and other disease in which peptide deformylases are involved, especially in the treatment of bacterial and parasitic infections, for example infections fully or partly caused by microorganisms belonging to Staphylococcus, Enterococcus, Streptococcus, Haemophilus, Moraxella, Escherichia, Mycobacterium, Mycoplasma, Pseudomonas, Chlamydia, Rickettsia, Klebsiella, Shigella, Salmonella, Bordetella, Clostridium, Helicobacter, Campylobacter, Legionella or Neisseria.
Owner:ARPIDA AG
Recombinant high molecular weight major outer membrane protein of Moraxella
An isolated and purified outer membrane protein of a Moraxella strain, particularly M. catarrhalis, having a molecular mass of about 200 kDa, is provided by recombinant means. The about 200 kDa outer membrane protein as well as nucleic acid molecules encoding the same are useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a bacterial pathogen that produces the about 200 kDa outer membrane protein or produces a protein capable of inducing antibodies in a host specifically reactive with the about 200 kDa outer membrane protein.
Owner:LOOSMORE SHEENA +3
Moraxella bovis cytotoxin, cytotoxin gene, antibodies and vaccines for prevention and treatment of moraxella bovis infections
Moraxella bovis cytotoxin, cytotoxin gene, antibodies and vaccines for prevention and treatment of Moraxella bovis infections Moraxella bovis cytotoxin and a gene encoding Moraxella bovis cytotoxin. Identification, isolation, cloning and identification of nucleotide sequence of the Moraxella bovis genes mbxA, mbxB, mbxC and mbxD, partial purification of the native cytotoxin, preparation of partially purified native and a recombinant Moraxella bovis cytotoxin, identification of an amino acid sequence of the cytotoxin, preparation of antibodies against the Moraxella bovis cytotoxin, preparation of vaccines against Moraxella bovis. Method for prevention and treatment of infectious bovine keratoconjunctivitis caused by Moraxella bovis.
Owner:RGT UNIV OF CALIFORNIA
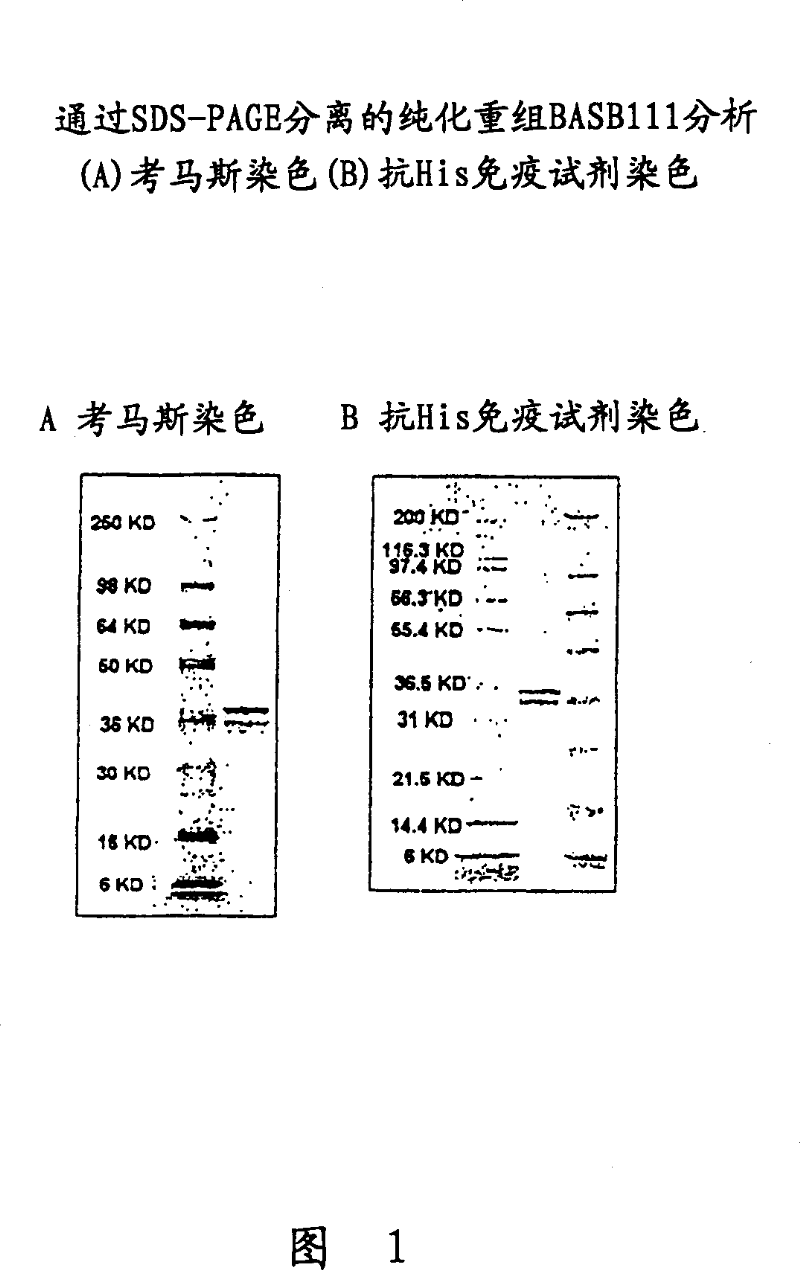
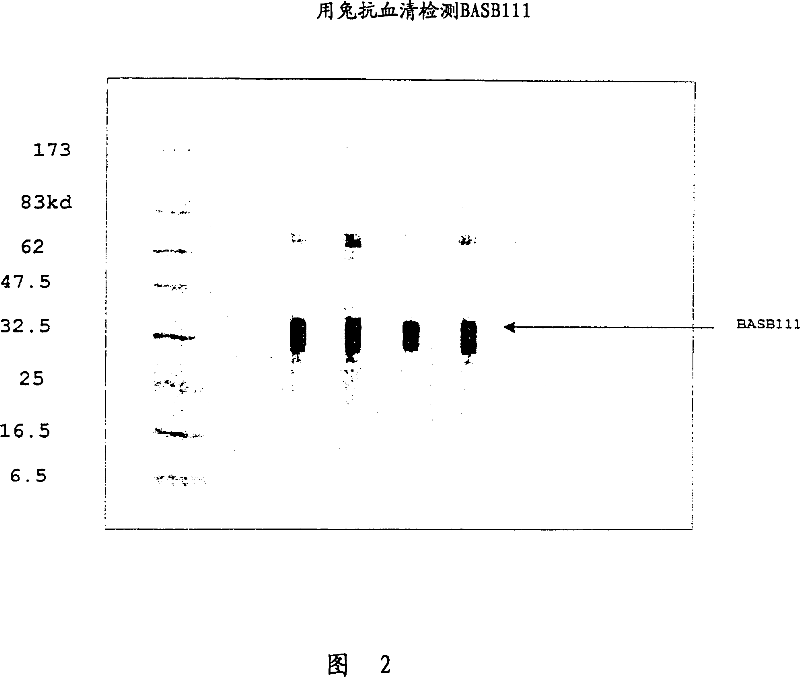
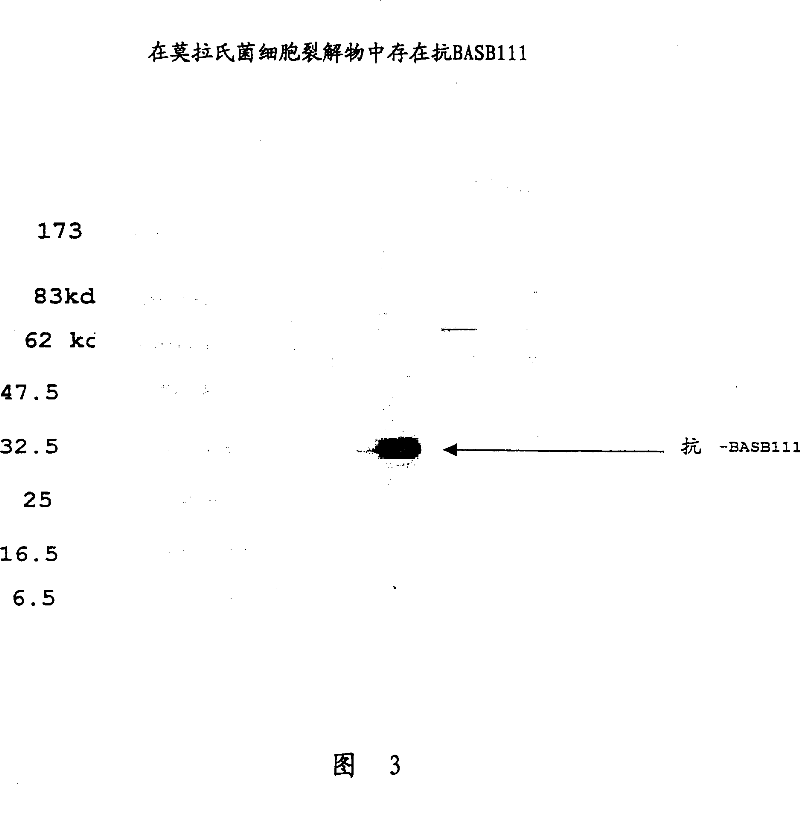
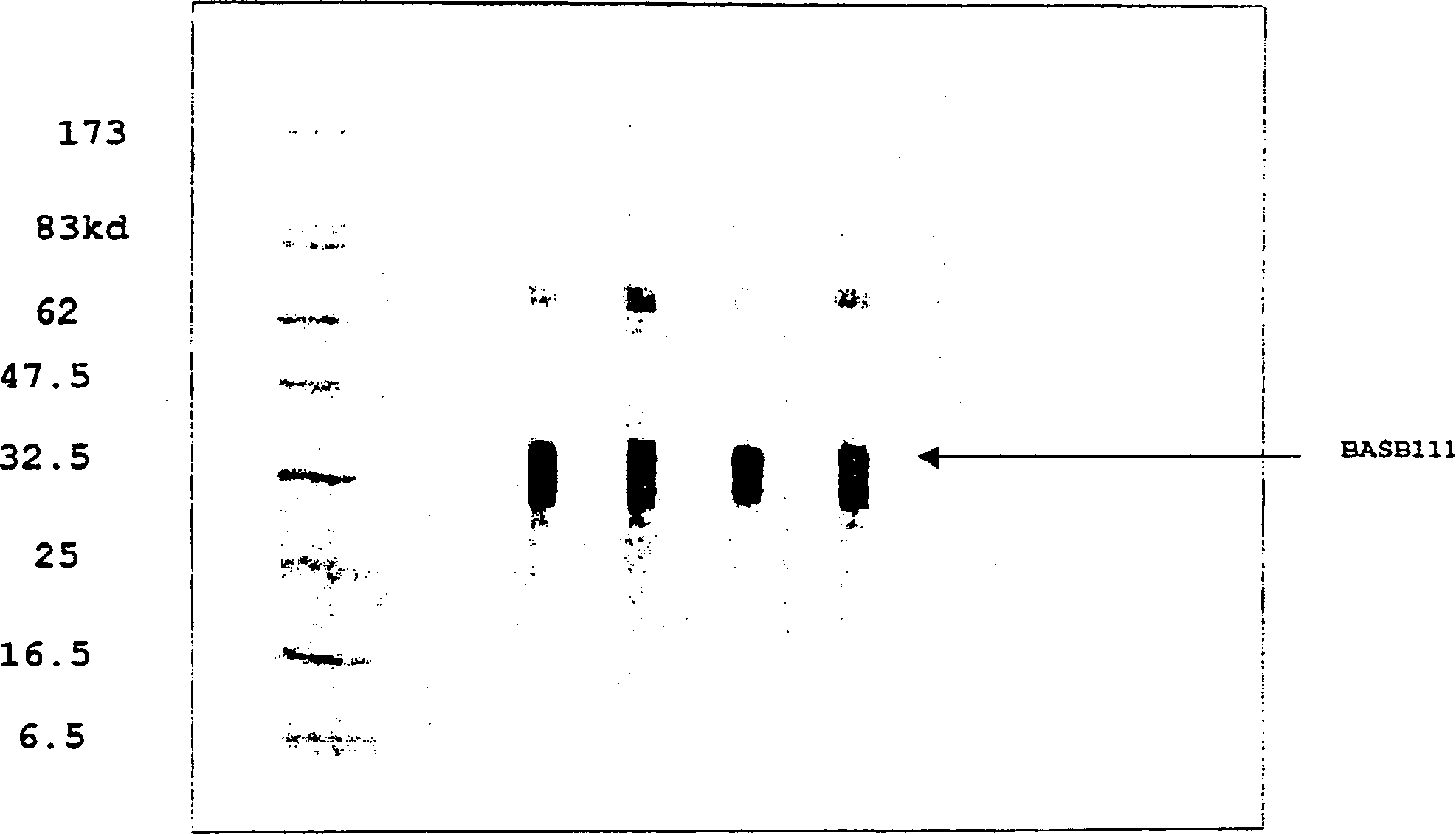
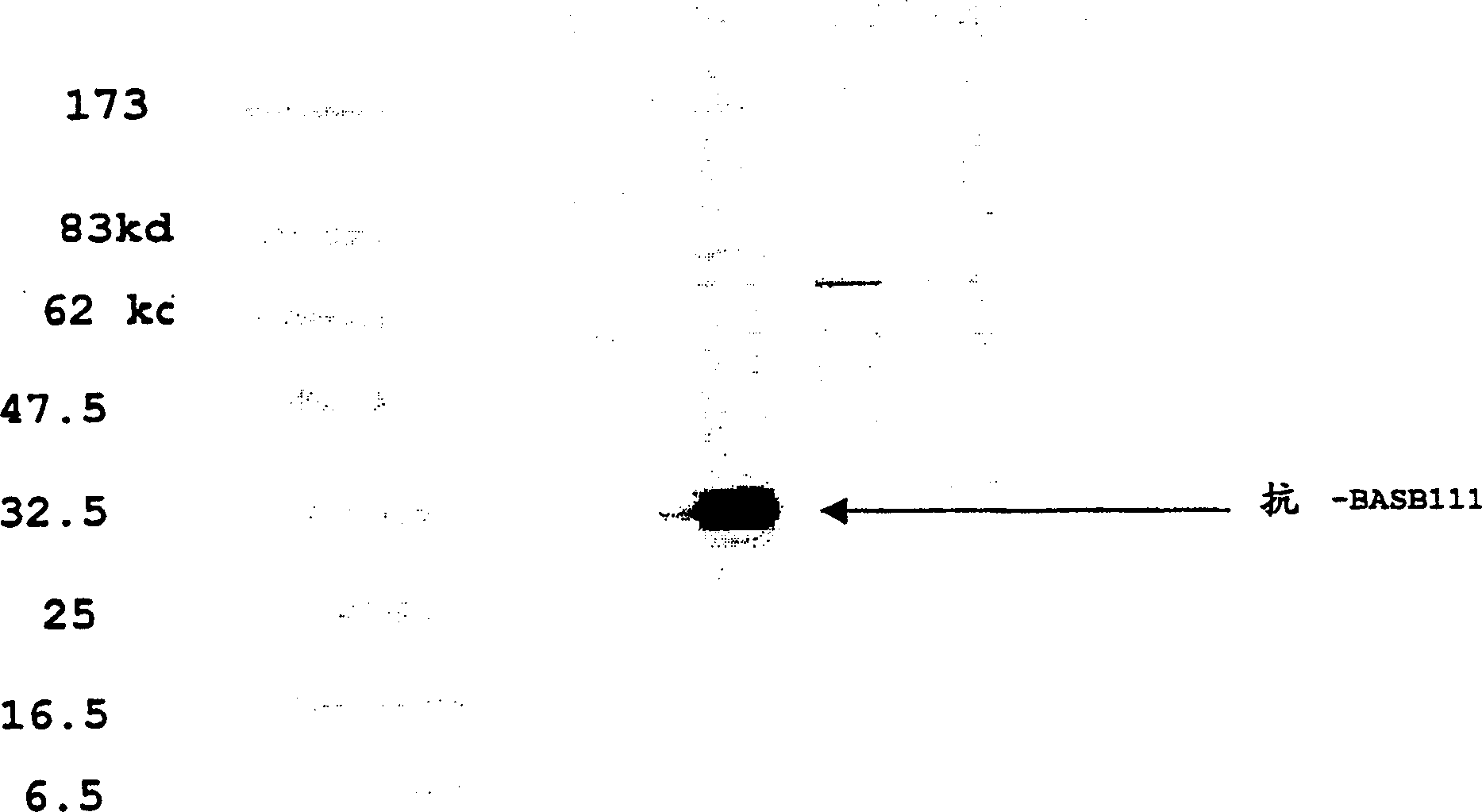
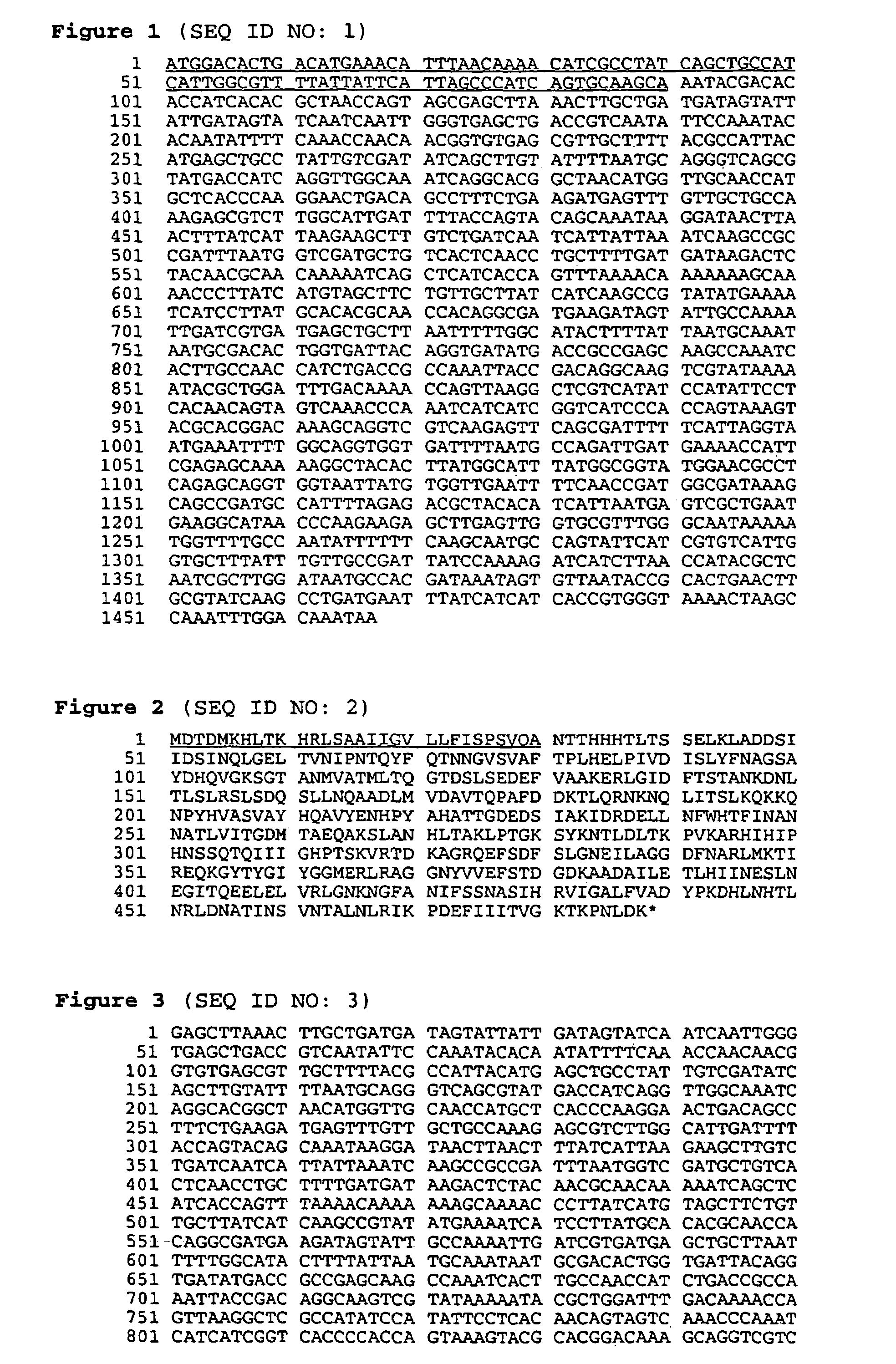
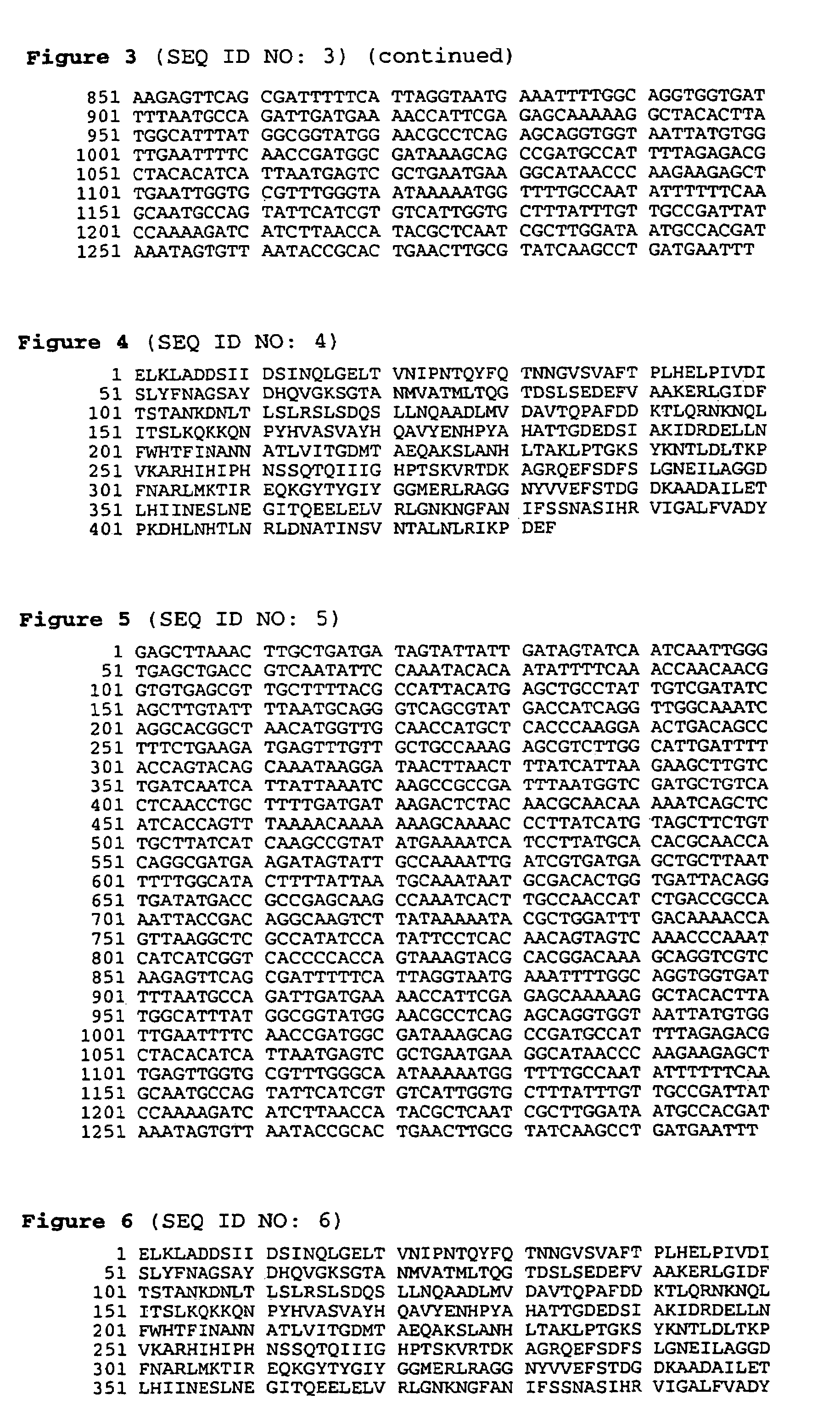
BASB 111 polypeptide and polynucleotide from moraxella cathraahalis
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
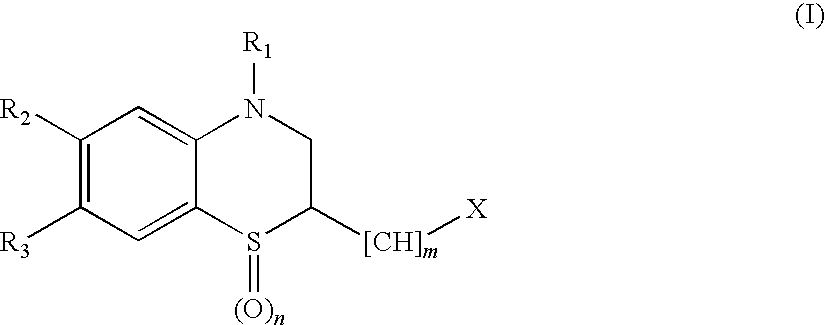
Peptide Deformylase Inhibitors
Benzothiazine compounds of the general formula (I) and pharmaceutically acceptable salts or esters thereof are peptide deformylase inhibitors useful in the treatment or prevention of infections and other diseases in which peptide deformylases are involved, especially in the treatment of bacterial and parasitic infections, for example infections fully or partly caused by microorganisms belonging to Staphylococcus, Enterococcus, Streptococcus, Haemophilus, Moraxella, Escherichia, Mycobacterium, Mycoplasma, Pseudomonas, Chlamydia, Rickettsia, Klebsiella, Shigella, Salmonella, Bordetella, Clostridium, Helicobacter, Campylobacter, Legionella or Neisseria.
Owner:ARPIDA AG
Bacterial extract for respiratory disorders and process for its preparation
ActiveUS8697154B2Improve bioavailabilityEfficient digestionBiocideBacteriaBacteroidesBacterial strain
The present invention relates to an extract from bacterial strains, such as Staphylococcus, Moraxella, Klebsiella, Streptococcus, and Haemophilus. The extract is useful as a treatment for indications such as respiratory disorders, compositions comprising the extract, and processes of making the extract from media that do not pose a risk of prion diseases.
Owner:OM PHARMA SA
Moraxella catarrahalis outer membrane protein-106 polypeptide, gene sequence and uses thereof
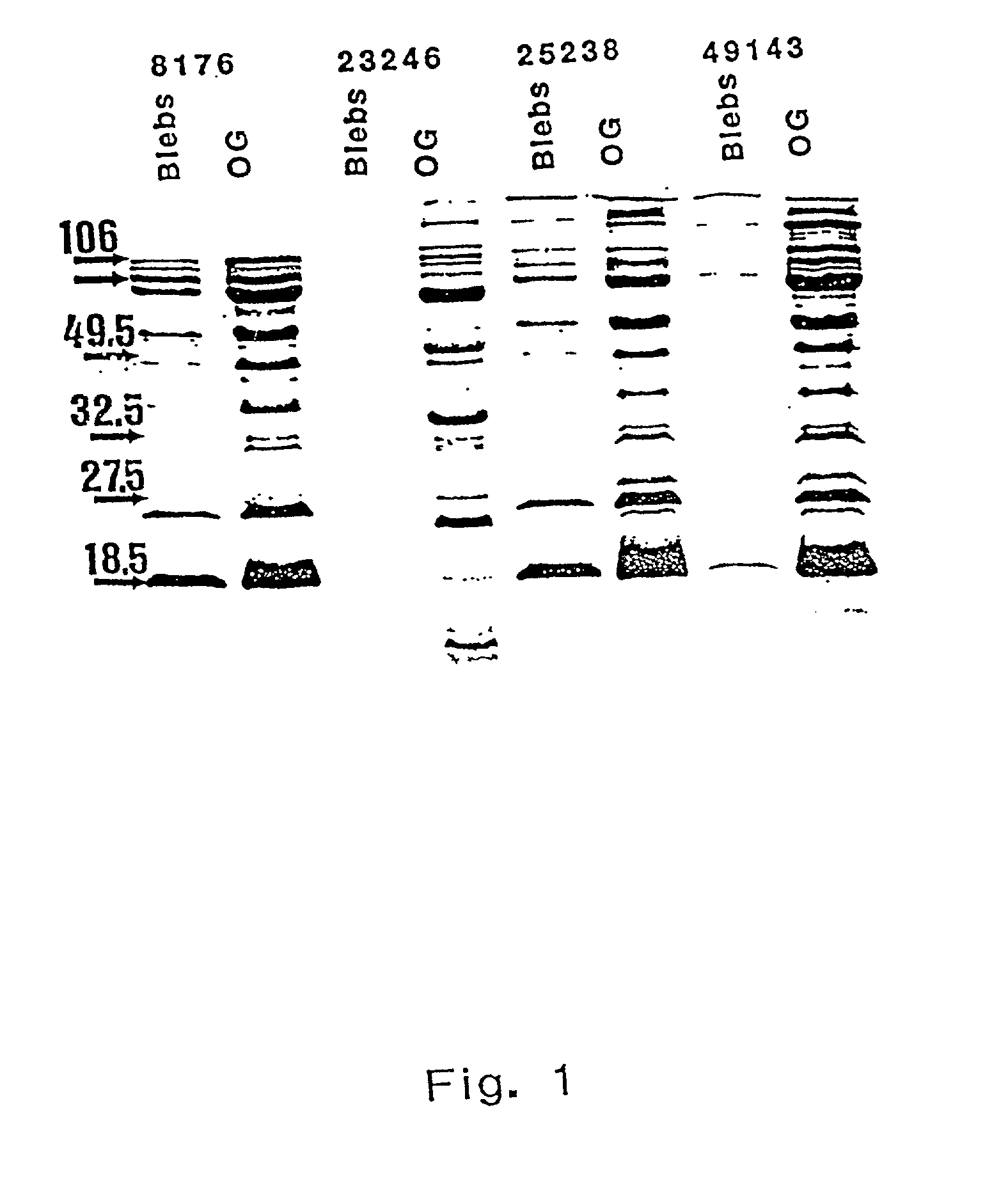
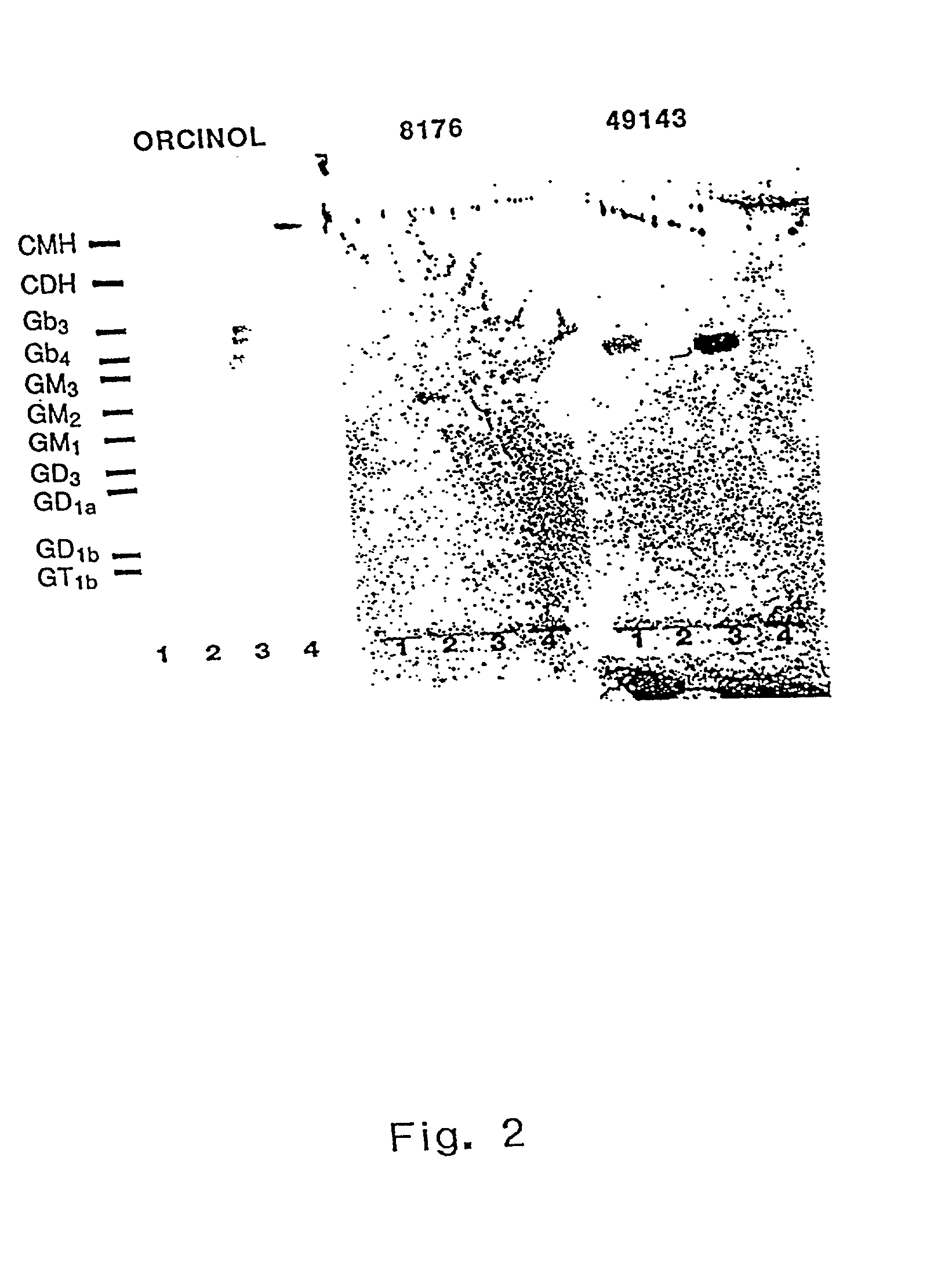
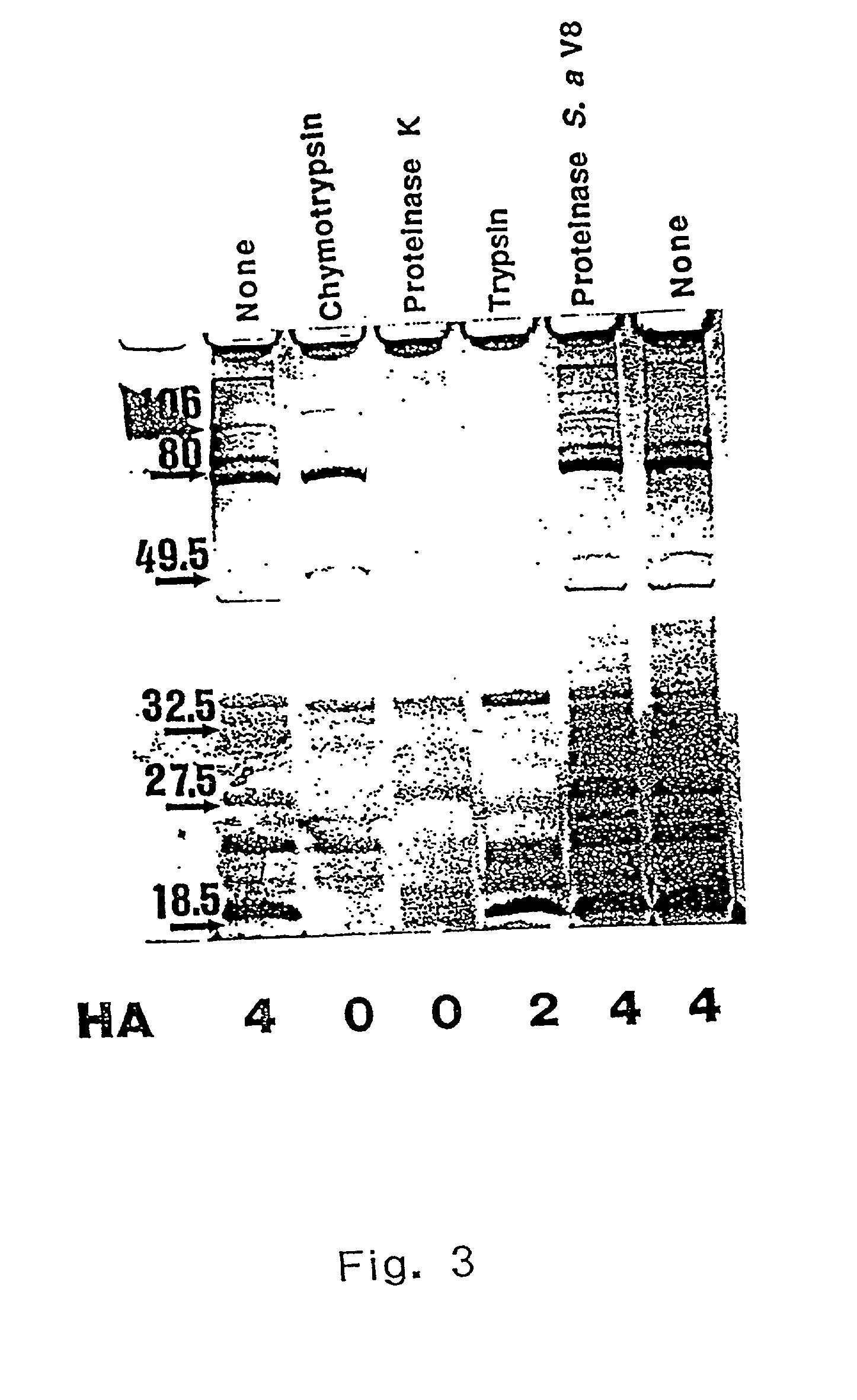
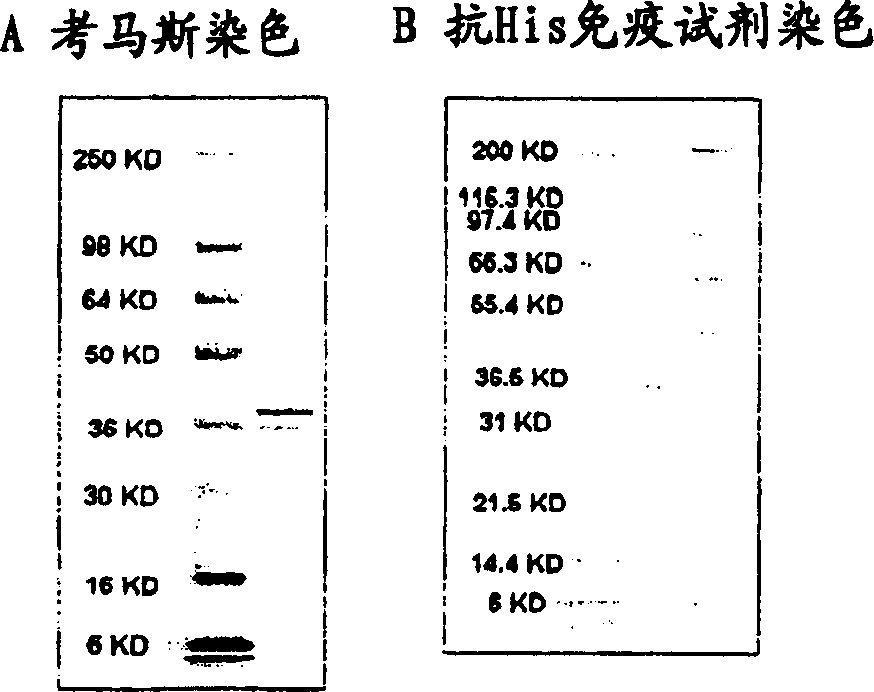
The invention discloses the Moraxella catarrhalis outer membrane protein-106 (OMP106) polypeptide, polypeptides derived therefrom (OMP106-derived polypeptides), nucleotide sequences encoding said polypeptides, and antibodies that specifically bind the OMP106 polypeptide and / or OMP106-derived polypeptides. Also disclosed are immunogenic, prophylactic or therapeutic compositions, including vaccines, comprising OMP106 polypeptide and / or OMP106-derived polypeptides. The invention additionally discloses methods of inducing immune responses to M. catarrhalis and M. catarrhalis OMP106 polypeptides and OMP106-derived polypeptides in animals.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
BASB 111 polypeptide and polynucleotide from moraxella cathraahalis
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Moraxella (Branhamella) catarrhalis antigens
Owner:ID BIOMEDICAL
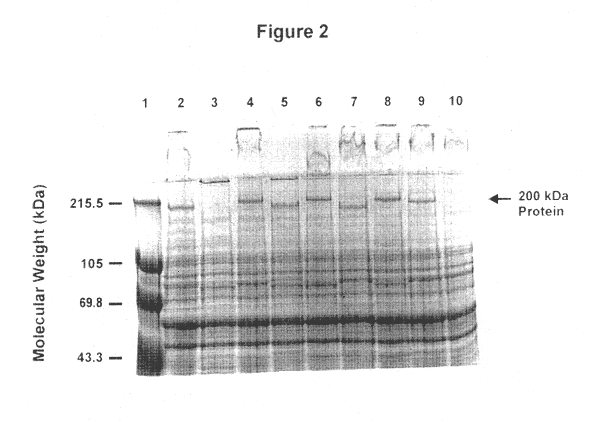
Recombinant high molecular weight major outer membrane protein of moraxella
An isolated and purified outer membrane protein of a Moraxella strain, particularly M. catarrhalis, having a molecular mass of about 200 kDa, is provided by recombinant means. The about 200 kDa outer membrane protein as well as nucleic acid molecules encoding the same are useful in diagnostic applications and immunogenic compositions, particularly for in vivo administration to a host to confer protection against disease caused by a bacterial pathogen that produces the about 200 kDa outer membrane protein or produces a protein capable of inducing antibodies in a host specifically reactive with the about 200 kDa outer membrane protein.
Owner:LOOSMORE SHEENA +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com