Moraxella (branhamella) catarrhalis polypeptides and corresponding DNA fragments
A DNA sequence and fragment technology, applied in the field of SMC-1 and SMC-2 polypeptides of Moraxella catarrhalis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
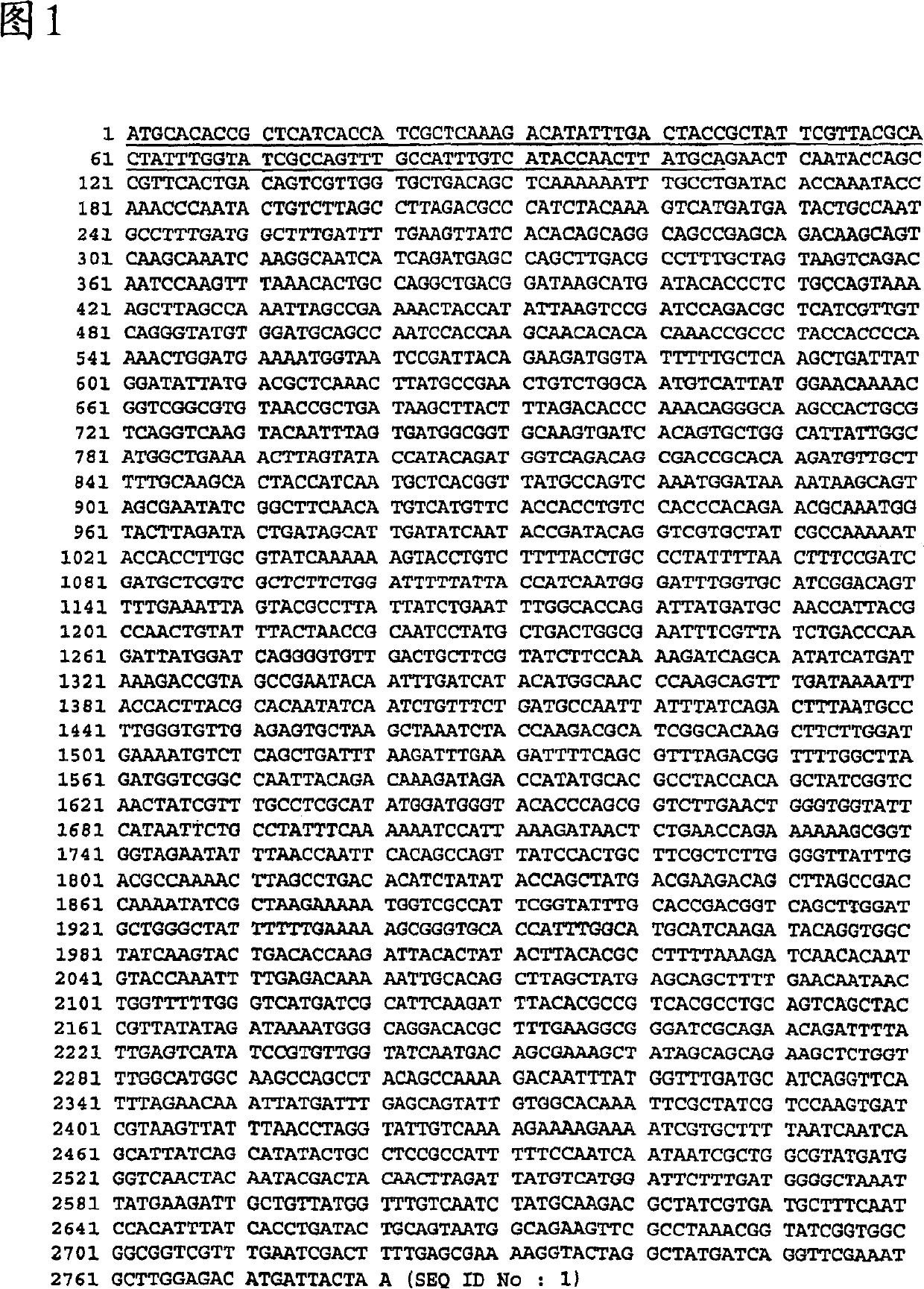
[0176] This example illustrates the cloning and molecular characterization of the SMC-1 gene and the corresponding polypeptide.
[0177] Moraxella catarrhalis SMC-1 (SEQ ID NO: 1) was amplified from Moraxella catarrhalis strain ETSU C-2 genomic DNA by PCR method (DNAThermal Cycler GeneAmp PCR system 2400 Perkin Elmer, San Jose, CA) The coding region was amplified using oligonucleotides containing base extensions for the addition of restriction sites NcoI (CCATGG) and XhoI (CTCGAG): RIOS30 (5'-TATGTACCATGGCTGAACTCAATACCAGCCGTTCA-3') and RIOS31 ( 5'-GGCATGCTCGAGGTAATCATGTCTCCAAGCATTTTG-3'). PCR products were purified from agarose gels using the QIAquick Gel Extraction Kit (Qiagen, Chatsworth, CA) according to the manufacturer's instructions and digested with NcoI and XhoI (Amersham PharmaciaBiotech, Inc, Baie d'Urfé, Canada). The pET21d(+) vector (Novagen, Madison, WI) was digested with Ncol and XhoI and the product was purified from agarose gel using the QIAquick Gel Extractio...
Embodiment 2
[0183] This example illustrates the cloning and molecular characterization of the SMC-2 gene and the corresponding polypeptide. Moraxella catarrhalis SMC-2 (SEQ ID NO: 3) was amplified by PCR method (DNA ThermalCycler GeneAmp PCR system 2400 Perkin Elmer, San Jose, CA) from Moraxella catarrhalis strain ETSU C-2 genomic DNA For the coding region, amplified using oligonucleotides containing a base extension for the addition of restriction sites NcoI (CCATGG) and XhoI (CTCGAG): RIOS20 and RIOS21, both of which are listed in Table 1 out. The method for cloning the SMC-2 gene into an expression vector and performing sequencing is similar to the method described in Example 1.
[0184] It has been determined that the open reading frame (ORF) encoding SMC-2 contains 957-bp (base pairs) and encodes a polypeptide with 318 amino acid residues. The predicted pI is 5.78 and the predicted molecular weight is 35954.10Da. Using Spscan software (Wisconsin Sequence AnalysisiPackage; Genetics ...
Embodiment 3
[0187] This example illustrates the cloning of M. catarrhalis genes in the CMV plasmid pCMV-GH.
[0188] In-phase insertion of the DNA coding region of the Moraxella catarrhalis polypeptide into the vector pCMV-GH (Tang et al., Nature, 1992, 356:152) Human growth under the transcriptional control of the plasmid cytomegalovirus (CMV) promoter Downstream of the hormone (hGH) gene. The CMV promoter was not functional in E. coli cells, but was active when administered to eukaryotic cells. The ampicillin resistance gene was also inserted into the vector.
[0189] From the genomic DNA of Moraxella catarrhalis strain ETSU C-2, the SMC-1 (SEQ ID NO: 1) and SMC-2 (SEQ ID NO: 3) coding regions without the leader peptide were amplified by PCR to amplify Oligonucleotides containing a base extension for the addition of restriction sites BamHI (GGATCC), BglII (AGATCT), SalI (GTCGAC) or HindIII (AAGCTT) were used, listed in Table 1. PCR products were purified from agarose gels using the Q...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com