Moraxella bovis cytotoxin, cytotoxin gene, antibodies and vaccines for prevention and treatment of moraxella bovis infections
a technology of moraxella bovis and cytotoxin, which is applied in the field of moraxella bovis cytotoxin and a gene encoding moraxella bovis cytotoxin, can solve the problems of insufficient control measures that could substantially reduce this expense, the potential for adulteration of the nation's food supply, and the success of antimicrobial treatment of ibk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Moraxella bovis Bacteria
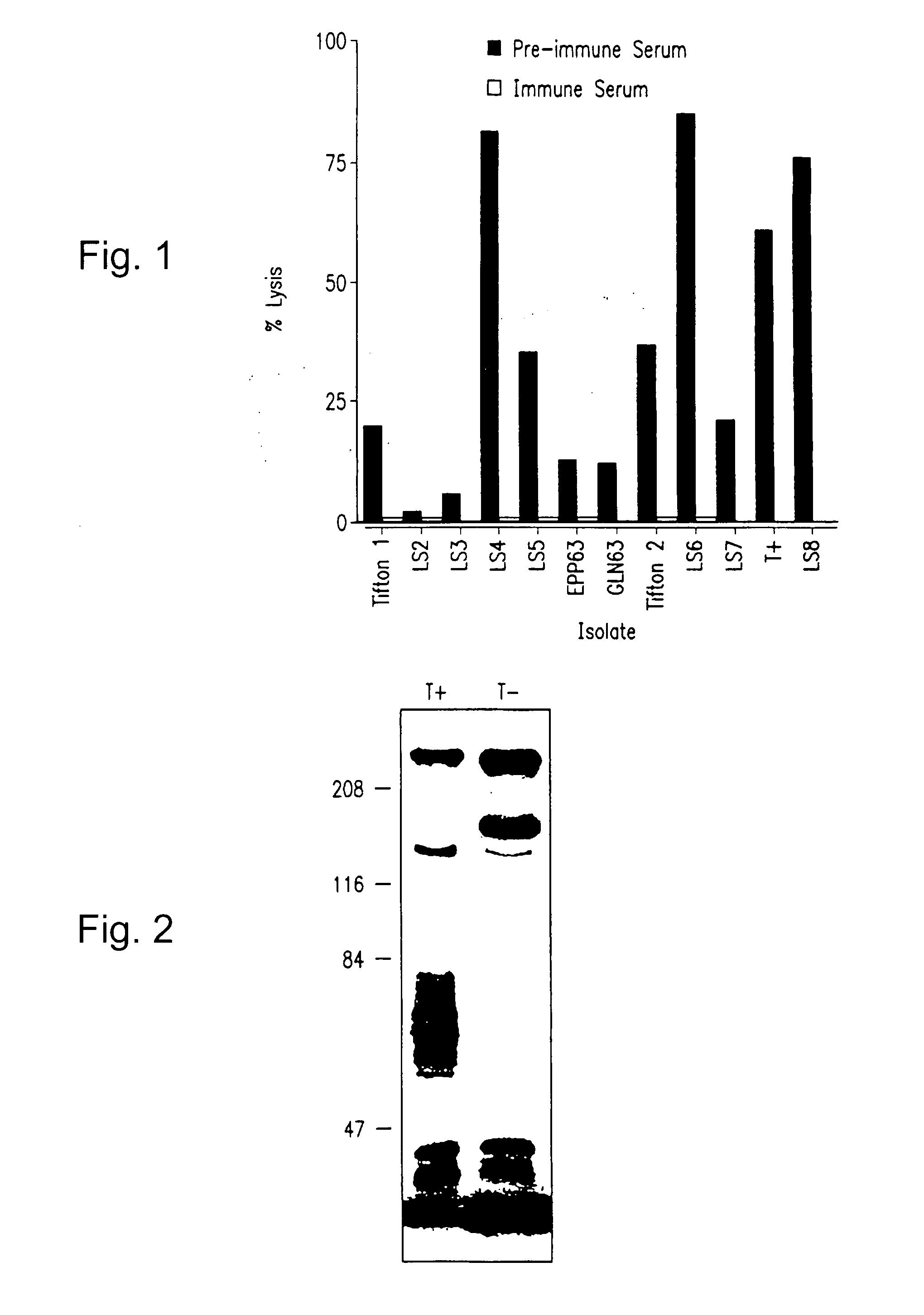
[0193] This example describes isolation of a cytolytic strain of Moraxella bovis T+ and a noncytolytic subculture T−.
[0194] A hemolytic, pathogenic strain of Moraxella bovis (T+) was isolated from a beef cow with infectious bovine keratoconjunctivitis. Cytolytic EPP and GLN 63 strains were furnished by Dr. G. W. Pugh (Ames, Iowa), and additional isolates were obtained from field specimens that were submitted to the laboratory for confirmation of bacterial identification. These additional isolates were recovered from clinically affected cattle located in regions of the United States that included southern Georgia (Tifton 1, Tifton 2), northern Georgia (GA 3), northern California (LS-2, LS-4, LS-6), southern California (LS-3, LS-8), Washington (LS-5), and North Carolina (LS-7). Isolate were identified and propagated as described in Acta Path. Microbiol. Scand., 80B, 629-640 (1972) and in Identification of Non-Enteric Gram-Negative Bacteria, US De...
example 2
Purification and Concentration of the Cytotoxin
[0195] This example describes the procedure used for purification and concentration of the cytotoxin when produced in M. bovis shaker cultures.
[0196] The cytotoxin was produced in broth shaker cultures that were inoculated with lawn cultures of M. bovis. The inoculum was harvested from the surface of 10, 20 hour sheep blood agar plate lawns of M. bovis with sterile cotton tipped applicators. The bacteria on the swab were suspended into 10 ml of heart infusion broth (Difco Laboratories, Detroit, Mich.) spiked with 1.5 mM CaCl2.
[0197] Flasks were inoculated with the suspension, and were incubated at 35.5±0.5° C. on a rotary shaker set at 200 oscillations per minute. The cultures were removed from the incubator when the optical densities (420 nM) reached 1.85. For purification, the cultures (living whole cells) were centrifuged for one hour at 13,000×g (4° C.) and the supernatants (centrifuged supernatants) were filtered through a steri...
example 3
Cytotoxin Preparation for Neutralization Studies
[0202] This example describes the procedure used for preparation of cytotoxin for neutralization studies.
[0203]Moraxella bovis lawns were grown on trypticase soy agar plates supplemented with 5% sheep blood. After 24 hours of incubation, the bacterial cells were harvested by flooding the plate with 10 ml of Tris buffered saline solution (TBS CaCl2 50 mM Tris, 150 mM NaCl, 1.5 mM CaCl2, pH 8.0), and suspending the bacterial growth with a sterile inoculating loop. The suspensions were collected from the surface of the agar, and then were centrifuged at 27,000×G for one hour. After centrifugation, the supernatants were harvested, filtered through 0.22 μM polyethersulfone membrane (Gelman Biosciences, Ann Arbor, Mich.), and tested for cytolytic activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com