Ophthalmic fluid delivery system
a fluid delivery and ophthalmic technology, applied in the field of ophthalmic fluid delivery system, can solve the problems of inefficient pharmacokinetics, sub-therapeutic drug levels, and the means of ophthalmic drug delivery, and achieve the effect of removing the drawbacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
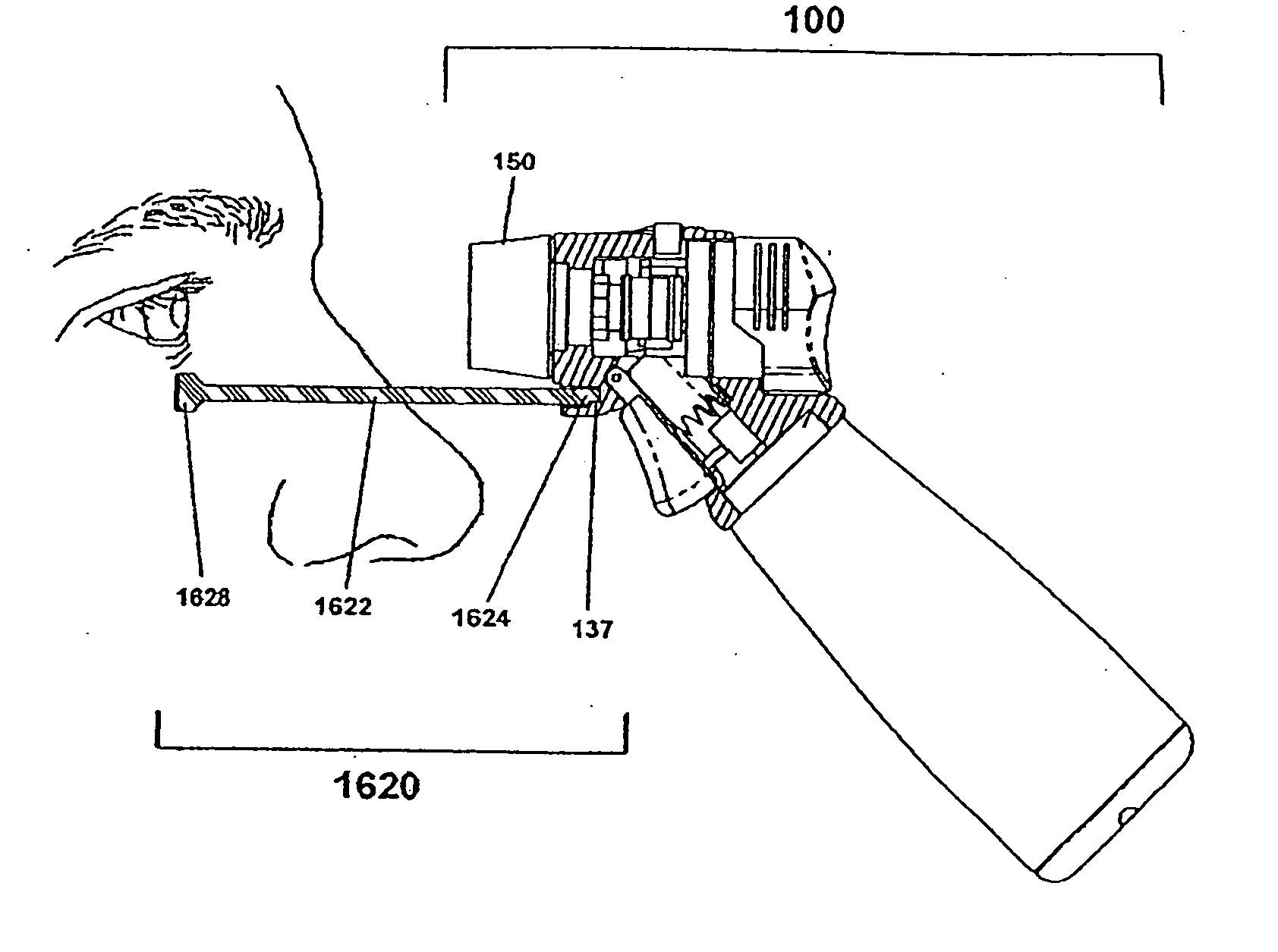
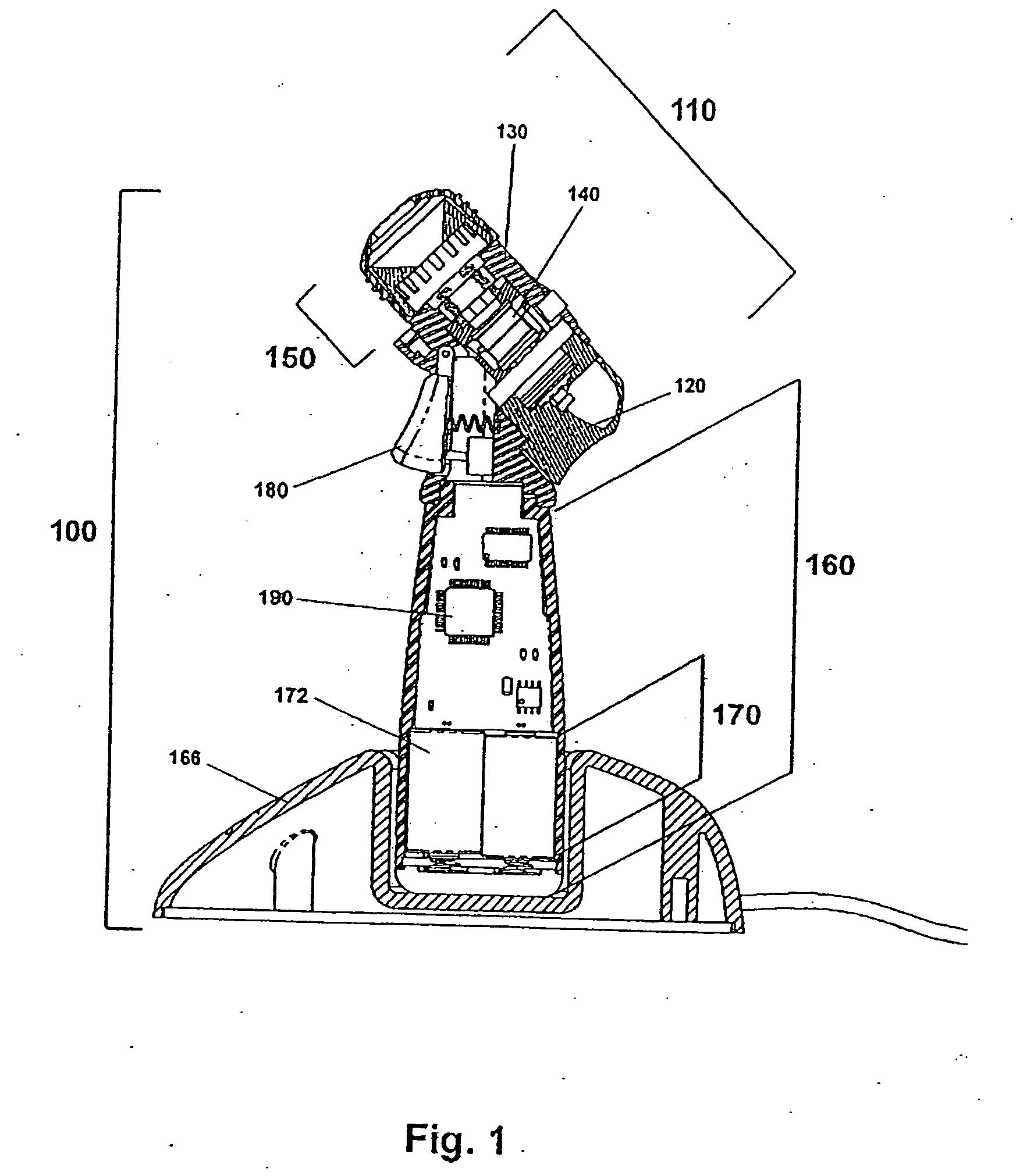
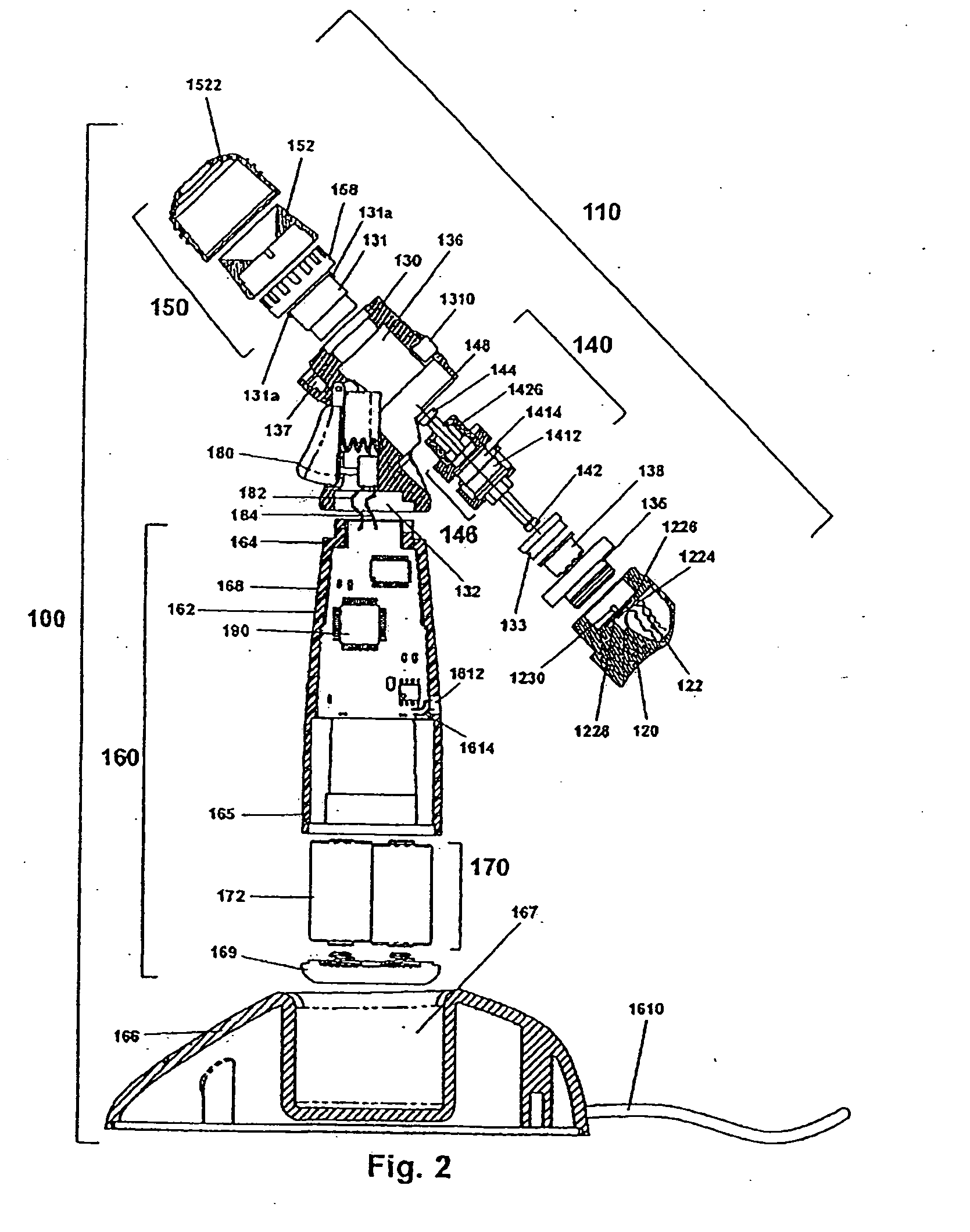
[0067]Certain terminology is used in the following description for convenience only and is not limiting. As used herein, the term “distal” is meant to mean the discharge end of the inventive device and the term “proximal” is meant to mean the end of the inventive device held by user. The terminology includes the words above specifically mentioned, derivatives thereof and words of similar import. The embodiments illustrated below are not intended to be exhaustive or to limit the invention to the precise form disclosed. These embodiments are chosen and described to best explain the principle of the invention and its application and practical use and to enable others skilled in the art to best utilize the invention.
[0068]The present invention provides a novel device and method for ophthalmic drug delivery. In preferred embodiments, the present invention provides a small, hand-held, battery or ac powered device that nebulizes liquid eye medications into a fine mist. The mist from the de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com