Stabilization and ionic triggering of nitric oxide release
a technology of nitric oxide and ionic triggering, which is applied in the direction of nitric oxide, chemistry apparatus and processes, nitrogen oxide/oxyacids, etc., can solve the problems of fragile materials and limited use under restricted operating conditions, and achieve the effect of increasing or decreasing the ph of the reaction medium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
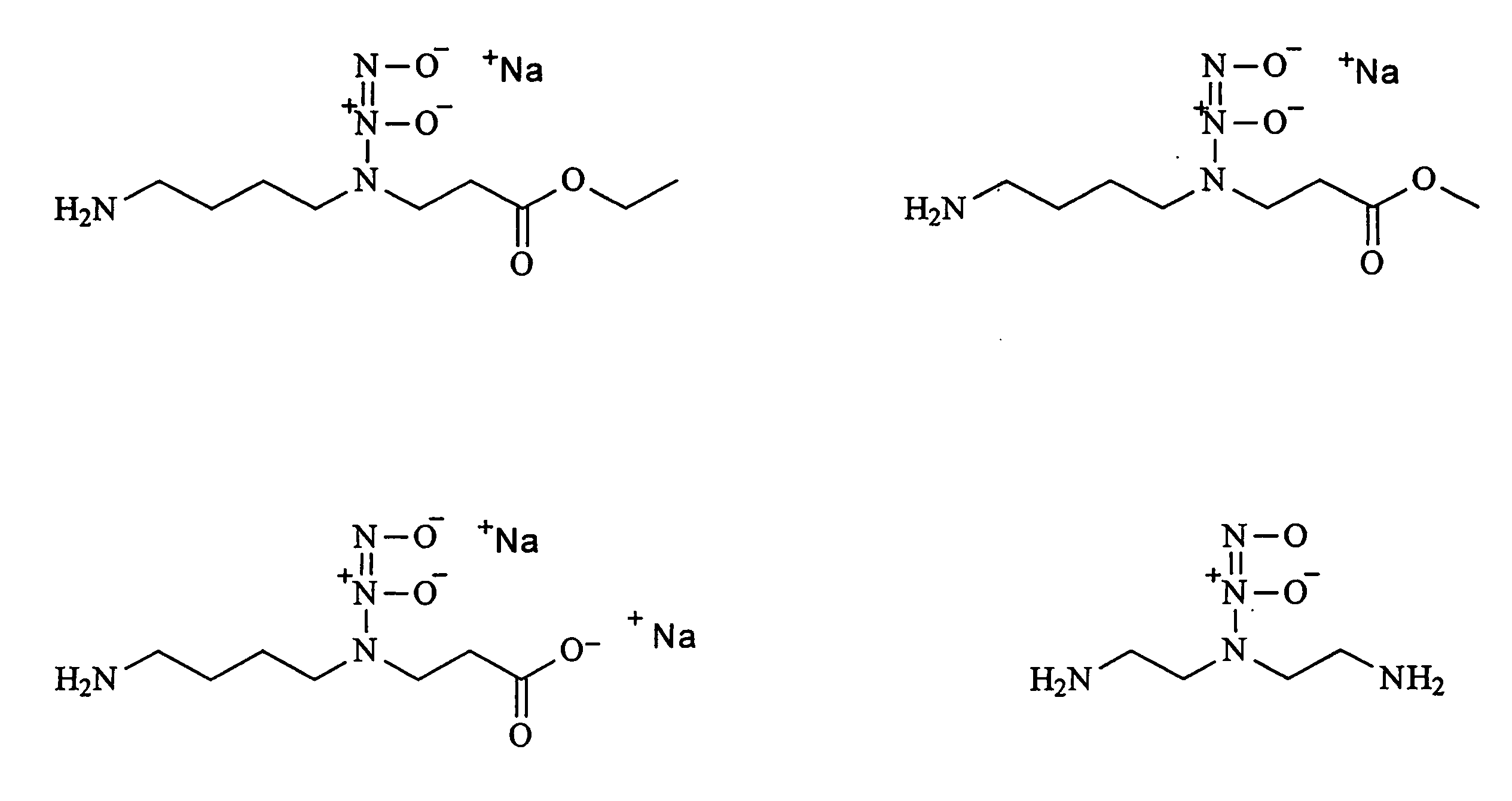
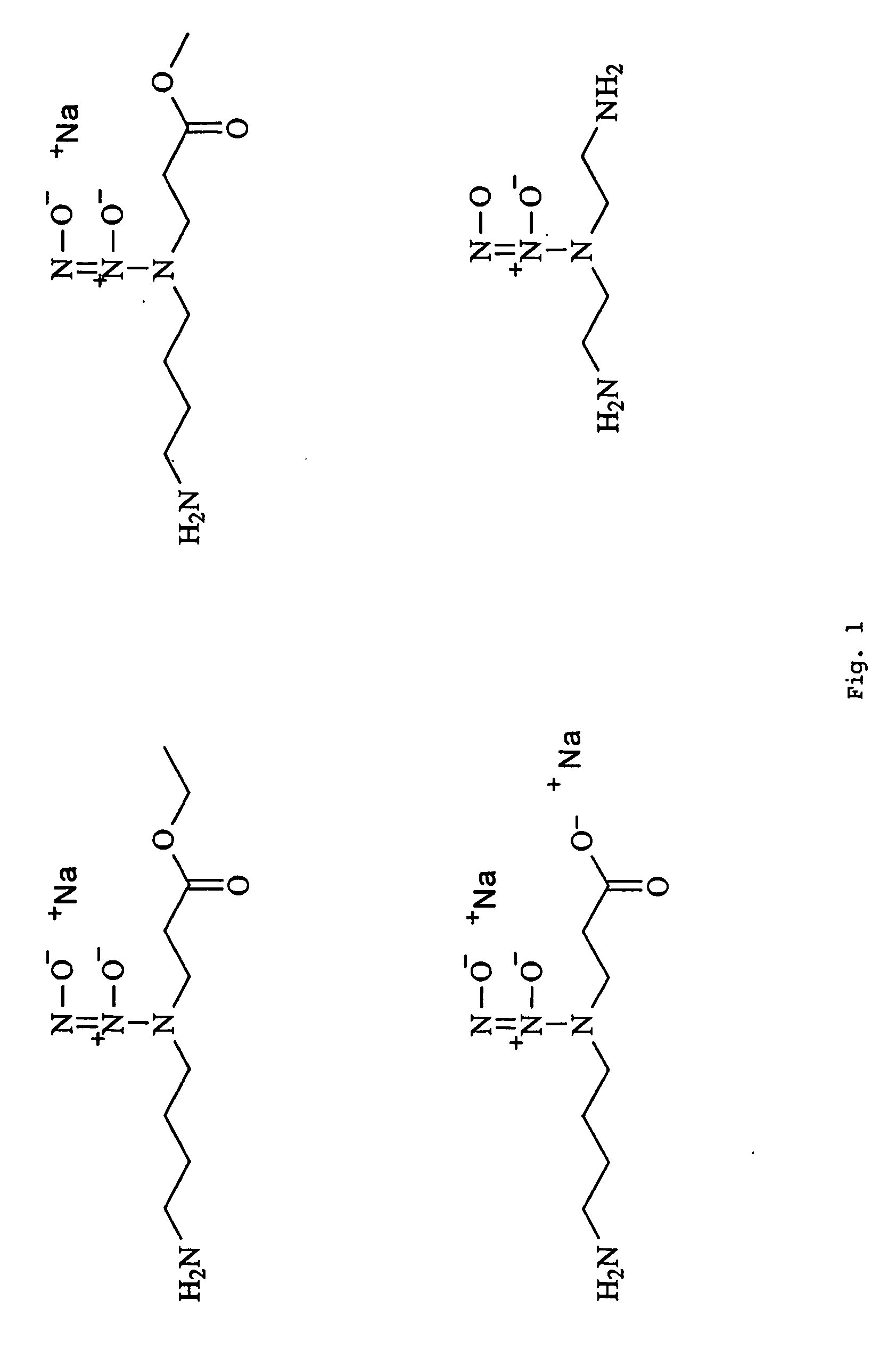
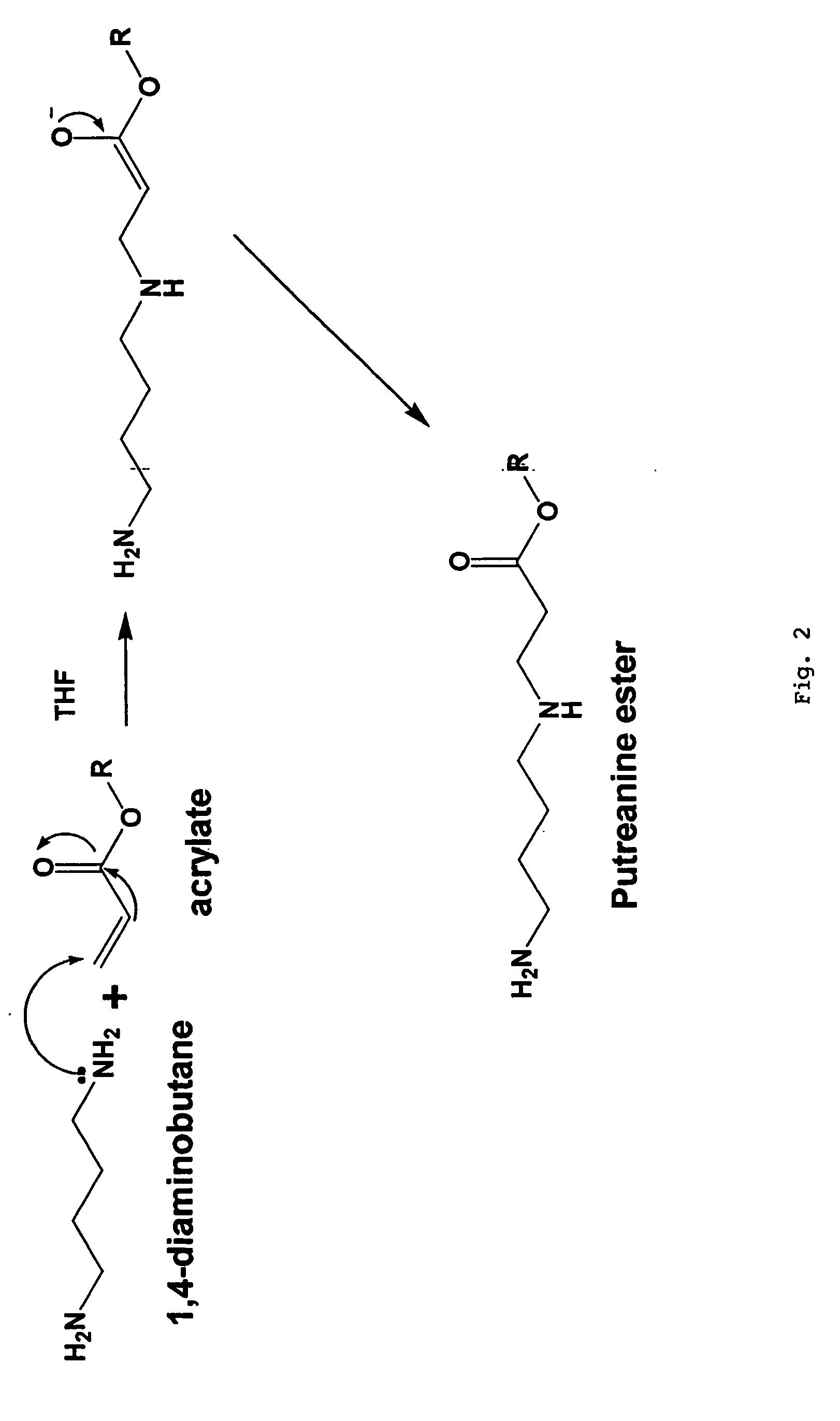
[0038]This invention is generally directed to a method for producing nitric oxide by using an ion exchange resin. The ionic exchange resin is either a cationic exchange resin or an anionic exchange resin. Using an ionic exchange resin allows a user to ionically trigger the release of anionic or cationic reactants from an ion exchange resin, whereby the anionic or cationic reactants preferably proceed to participate in producing nitric oxide via a chemical reaction.
[0039]This invention is further directed to producing NO by using a Ph adjuster used in combination with a diazeniumdiolate-containing composition.
[0040]This invention is further directed to mixing a salt with a cream, gel, or combination thereof to produce nitric oxide.
[0041]As mentioned above, an embodiment of this invention preferably employs an ionic exchange resin that forms an ion pair with a counter ion, wherein the counter ion can be displaced by a different and typically stronger cation or anion. There is no limit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com