Novel Carboxylesterase Nucleic Acid Molecules, Proteins and Uses Thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0178]This example describes labeling of proteases and esterases with radiolabeled diisopropylfluorophosphate.
[0179]Tissue samples were isolated from unfed or bovine blood-fed 1st instar Ctenocephalides felis flea larvae; bovine blood-fed or cat blood-fed 3rd instar Ctenocephalides felis flea larvae; bovine blood-fed or cat blood-fed Ctenocephalides felis prepupal flea larvae; bovine blood-fed or cat blood-fed adult Ctenocephalides felis flea midgut tissue, and whole unfed, bovine blood-fed or cat blood-fed adult Ctenocephalides felis fleas. The 1st instar, 3rd instar, prepupal and adult midgut tissues were then homogenized by freeze-fracture and sonicated in a Tris buffer comprising 50 mM Tris, pH 8.0 and 100 mM CaCl2. The whole adult flea sample was then homogenized by freeze-fracture and ground with a microtube mortar and pestle. The extracts were centrifuged at about 14,000×g for 20 minutes (min.) and the soluble material recovered. The soluble material was then diluted to a fin...
example 2
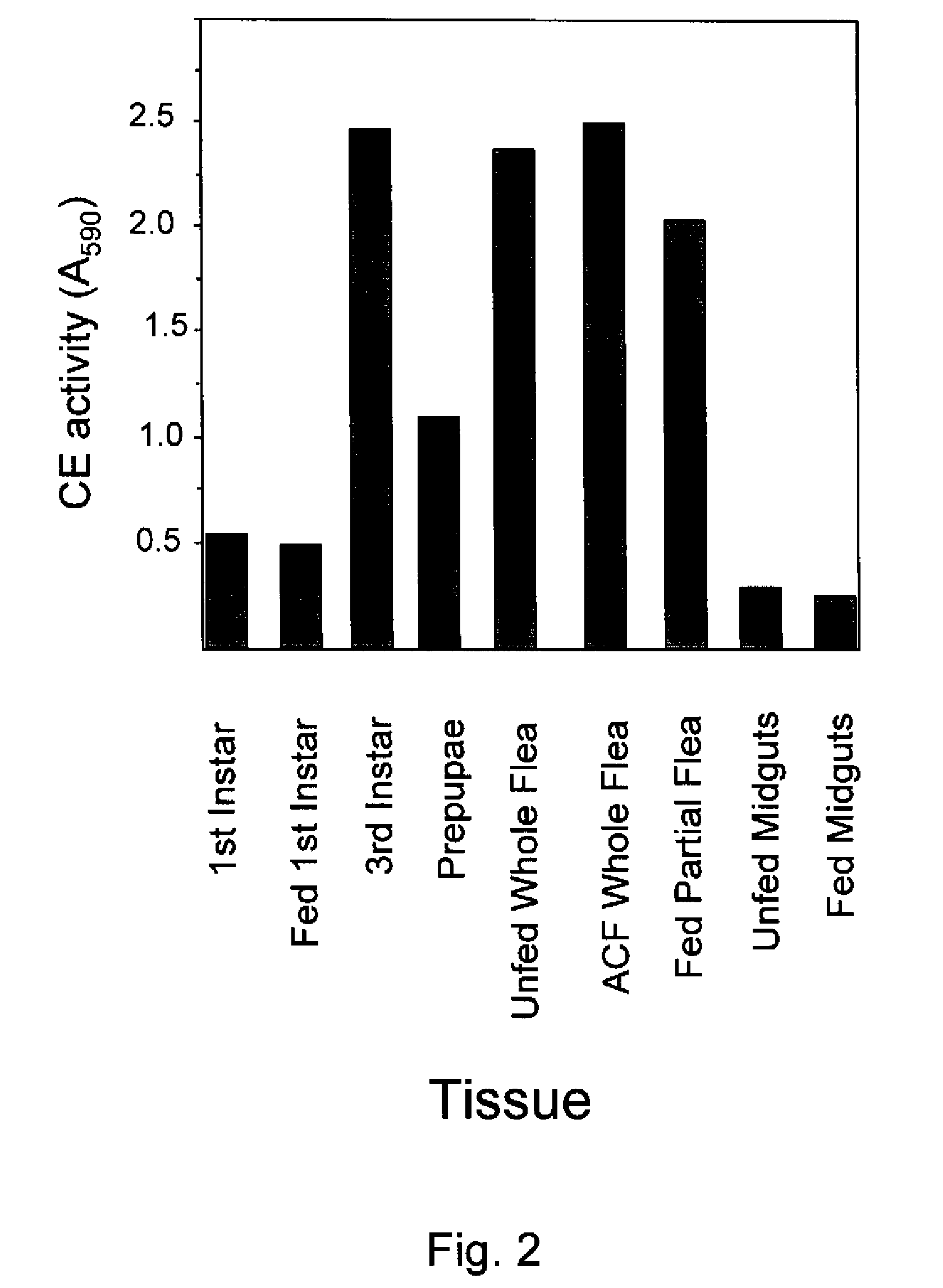
[0181]This example describes the identification of general CE activity in flea tissue extracts.
[0182]Tissue samples and soluble extracts were prepared as described above in Example 1, except not labelled, from unfed (UF) and bovine blood-fed 1st instar flea larvae, bovine blood-fed 3rd instar flea larvae, bovine blood-fed prepupal flea larvae, unfed whole adult fleas, cat blood-fed adult (ACF) whole fleas, cat blood-fed adult fleas that have had their heads and midguts removed (referred to herein as fed adult partial fleas), unfed adult flea midguts and cat blood-fed adult flea midguts. About 5 tissue equivalents of each tissue were assayed for general CE activity using the following method. Tissue samples of about 5 μl were added to separate wells of flat-bottomed microtiter plate (available from Becton Dickinson, Lincoln Park, N.J.). A control well was prepared by adding about 5 μl of Tris buffer to an empty well of the plate. About 95 μl of 25 mM Tris-HCl (pH 8.0) was then added ...
example 3
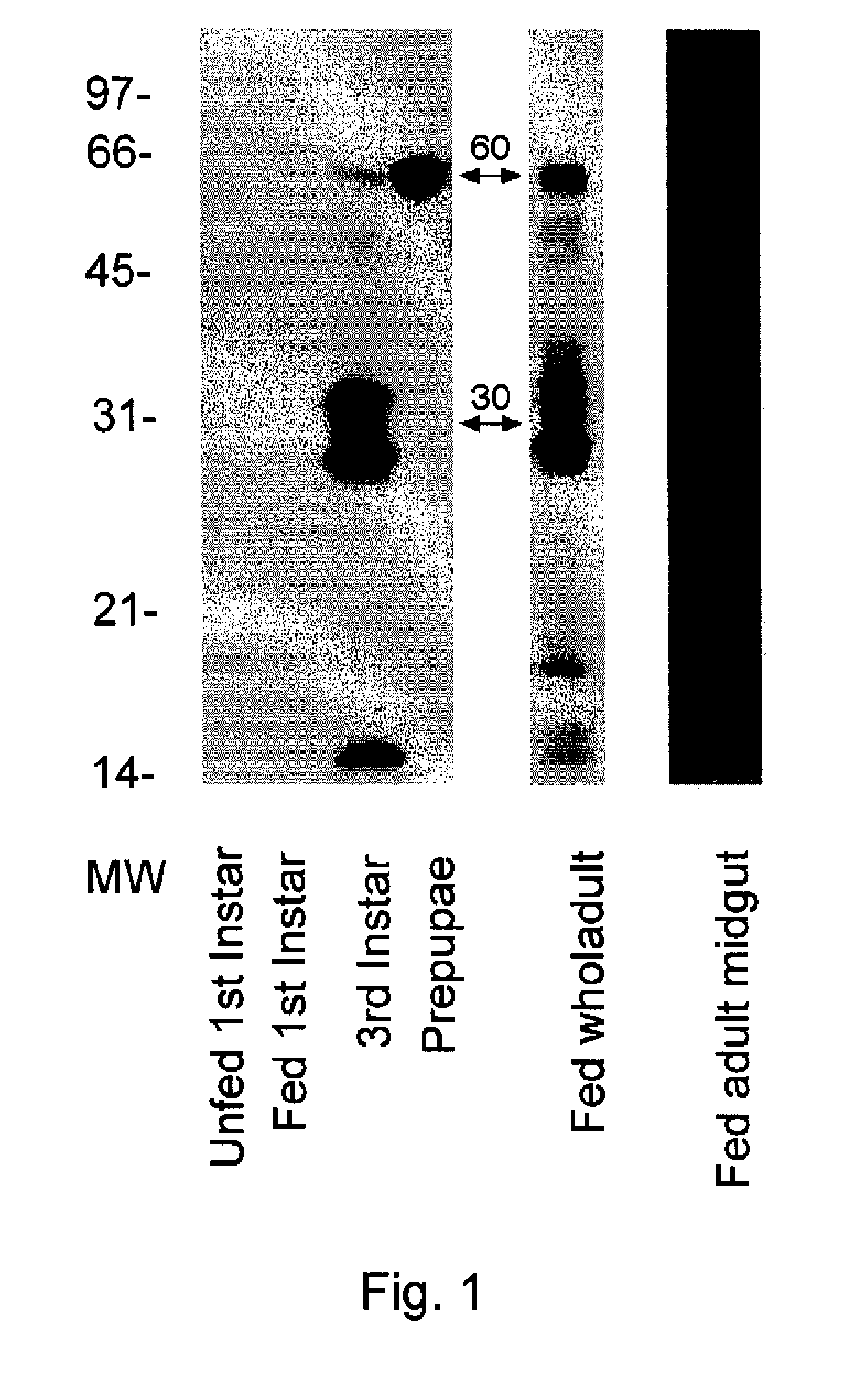
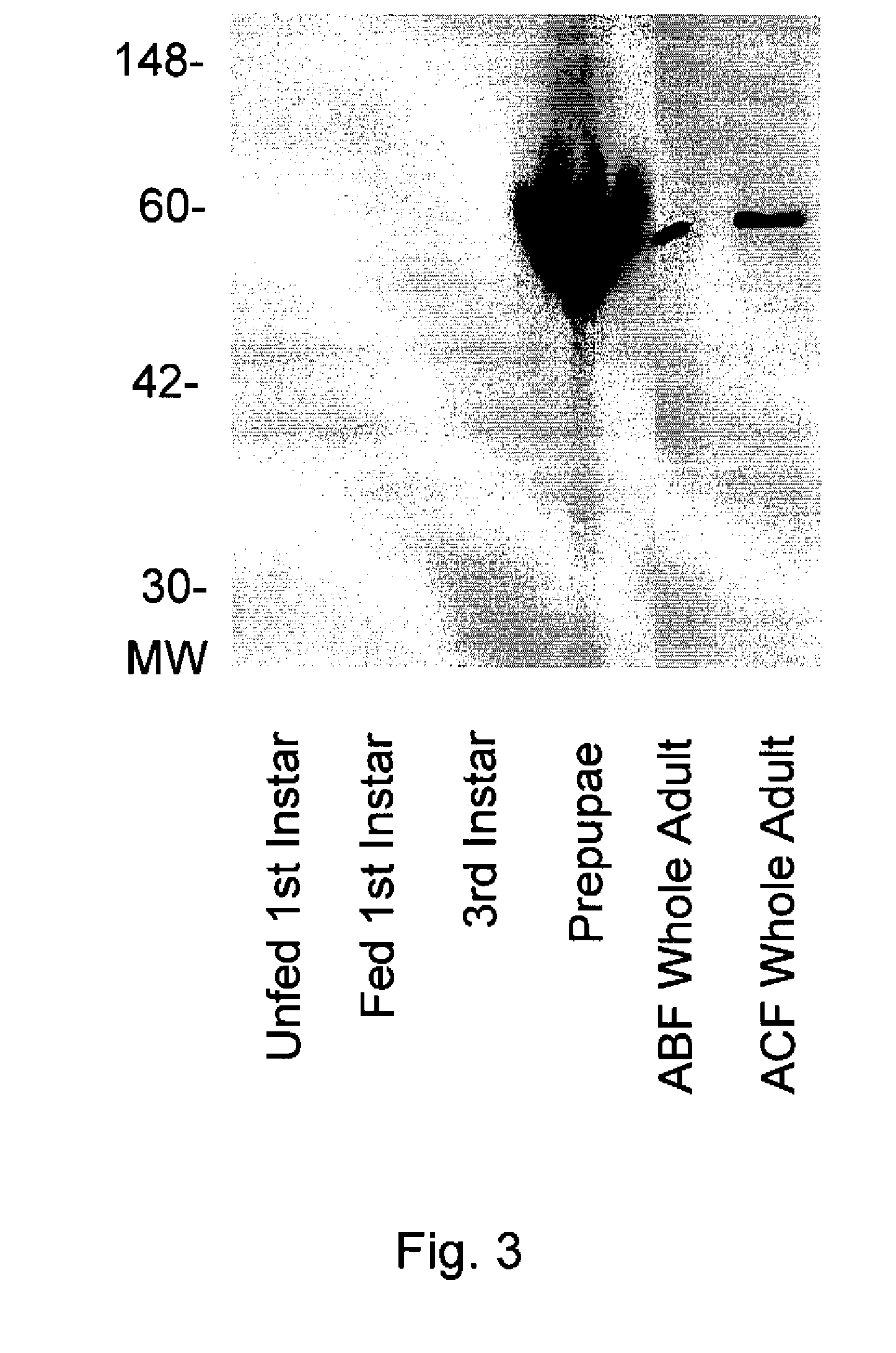
[0185]This example describes the determination of general CE activity using isoelectric focusing (JEF)-PAGE and non-reducing SDS-PAGE.
[0186]A. Non-Reducing SDS-PAGE.
[0187]Soluble extracts from unfed and bovine blood-fed 1st instar flea larvae, bovine blood-fed 3rd instar flea larvae, bovine blood-fed prepupal flea larvae, bovine blood-fed adult (ABF) whole fleas and cat blood-fed adult whole fleas were prepared using the method described in Example 1. Each soluble extract sample was combined with SDS sample buffer (available from Novex) and proteins in the samples were resolved by gel electrophoresis using 14% Tris-glycine SDS electrophoresis gels (available from Novex). The gels were run at room temperature for about 1 hour at 200 volts. After electrophoresis, the gels were soaked for about for 30 minutes in 50 mM Tris, pH 8.0, containing 2.5% Triton X-100 to renature the proteins. The gels were then soaked in 50 mM Tris, pH 8.0, for about 5 minutes and then stained for about 5 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com