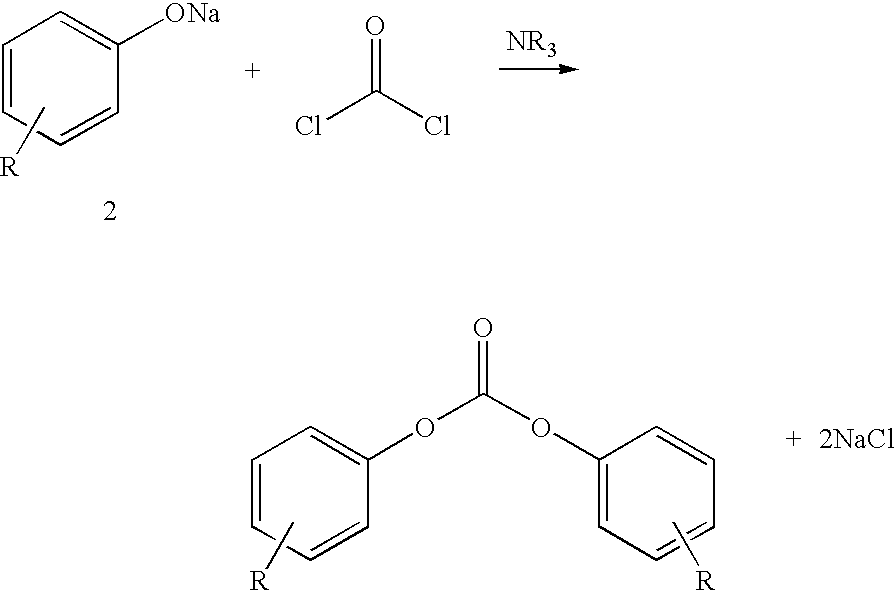
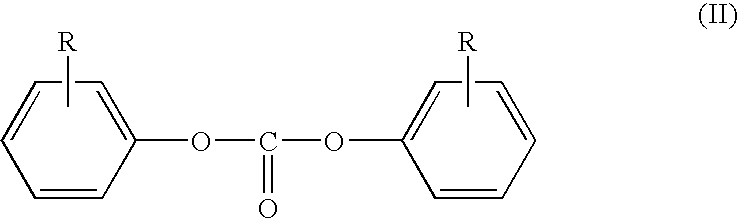
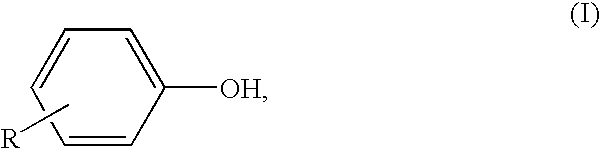Process for production of diaryl carbonate
a diaryl carbonate and production process technology, applied in the direction of carbonic/haloformic acid esters purification/separation, chlorine/hydrogen-chloride, phosgene or haloformate preparation, etc., can solve the problems of complex purification operation, inability to carry out the process economically, and high cost of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Addition of Enriched Sodium-Chloride-Containing Reaction Wastewater to the Sodium Chloride Electrolysis—Addition of a 22.5% Strength by Weight Sodium Chloride Solution from the DPC Production to the Sodium Chloride Electrolysis
[0111]a) Isolation of the Production Wastewater from DPC Production
[0112]In a vertically upright cooled tubular reactor, a mixture of 145.2 kg / h of 14.5% strength sodium hydroxide solution produced by diluting 65.8 kg / h of a 32% strength sodium hydroxide solution with 79.4 kg / h of demineralized water, and 48.3 kg / h of phenol was continuously combined with a solution of 86.2 kg / h of methylene chloride and 27.5 kg / h of phosgene (8 mol % excess based on phenol). This reaction mixture was cooled to a temperature of 33° C. and after a median residence time of 15 seconds, a pH of 11.5 was measured. Then, in the second stage of the process, to this reaction mixture was added 5.4 kg / h of 50% strength NaOH, so that the pH of the second reaction stage, after a further r...
example 2
Addition of Enriched Sodium-Chloride-Containing Production Wastewater to the Sodium Chloride Electrolysis—Addition of an 18.0% Strength by Weight Sodium Chloride Solution from the DPC Production to the Sodium Chloride Electrolysis
a) Sodium Chloride Enrichment of Production Wastewater by Wastewater Recycling to the Production Process
[0119]The procedure was followed as described in Example 1b) but the reaction wastewater was combined with the wash phases and subsequently freed from solvent residues and catalyst by stripping with steam. After neutralization with hydrochloric acid and treatment with activated carbon, the production wastewater contained 18.0% by weight NaCl and less than 2 ppm of organic impurities.
[0120]It could be fed without further purification to the electrochemical oxidation to chlorine.
b) Electrochemical Oxidation of the Enriched Production of Wastewater from a)
[0121]The electrolysis was carried out by way of example in a laboratory electrolysis cell having an ano...
example 3
Addition of Enriched Sodium-Chloride-Containing Reaction Wastewater to the Sodium Chloride Electrolysis—Addition of a 22.3% Strength by Weight Sodium Chloride Solution from DPC Production to the Sodium Chloride Electrolysis
a) Sodium Chloride Enrichment of Reaction Wastewater by Elevation of the Sodium Phenolate Concentration in the DPC Production
[0124]The procedure was followed as described in example 1a), but instead of 79.4 kg / h, only 54.6 kg / h of demineralized water was used. After separating off the organic phase from the aqueous phase (reaction wastewater), the organic phase was washed with 0.6% strength hydrochloric acid and water. After removal of the solvent, 99.9% pure diphenyl carbonate was obtained. The reaction wastewater, without prior combination with the wash phases, was freed from solvent residues and catalyst by stripping with steam. After neutralization with hydrochloric acid and treatment with activated carbon, the reaction wastewater contained 22.3% by weight NaC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com