Substantially animal protein-free recombinant furin and methods for producing same
a technology of recombinant furin and animal protein, which is applied in the field of recombinant furin and methods for producing the same, can solve the problems of inefficient processing and incomplete maturation, and achieve the effects of high specific activity, high purity and high specific activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of a Recombinant Furin Expression Plasmid and Host Cell Transfection
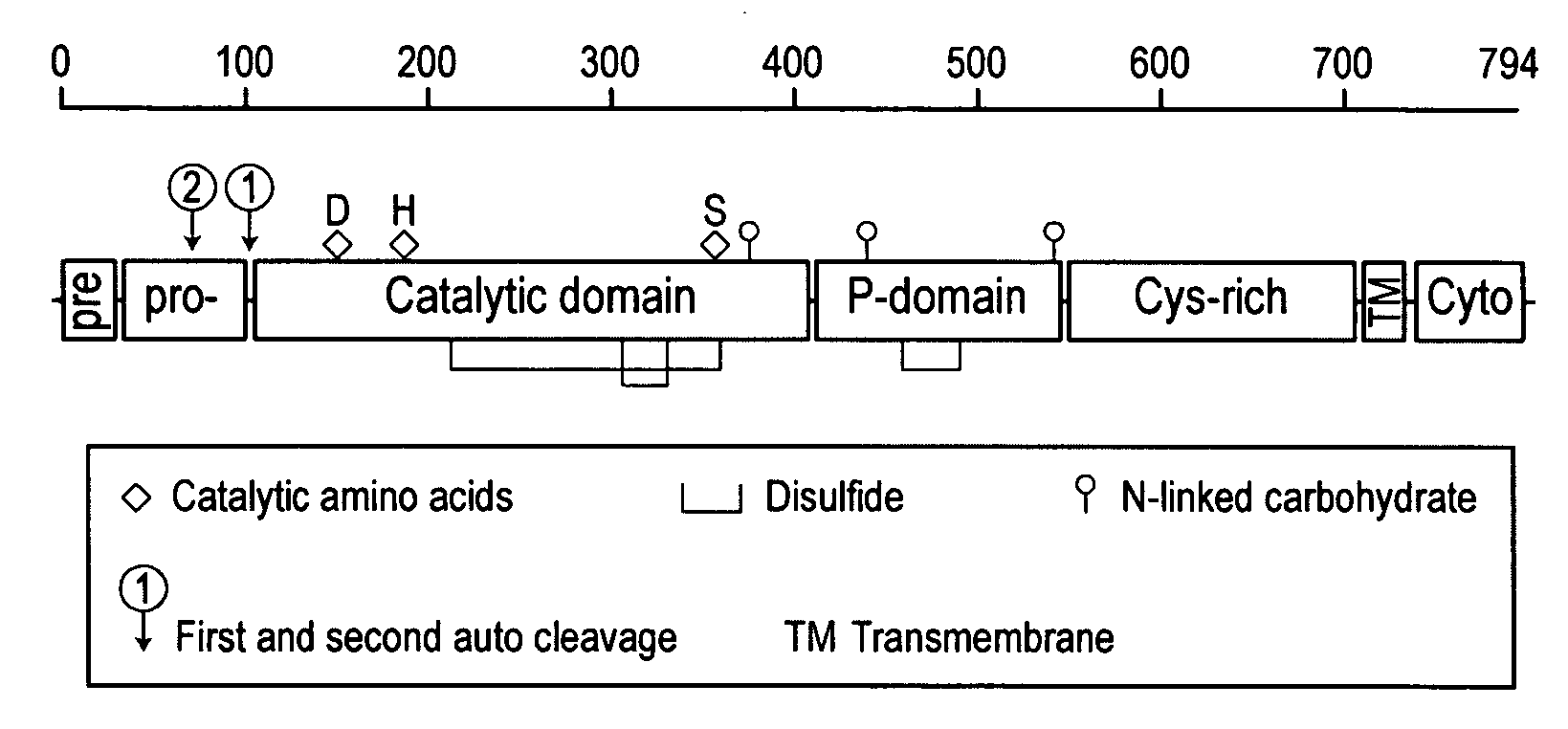
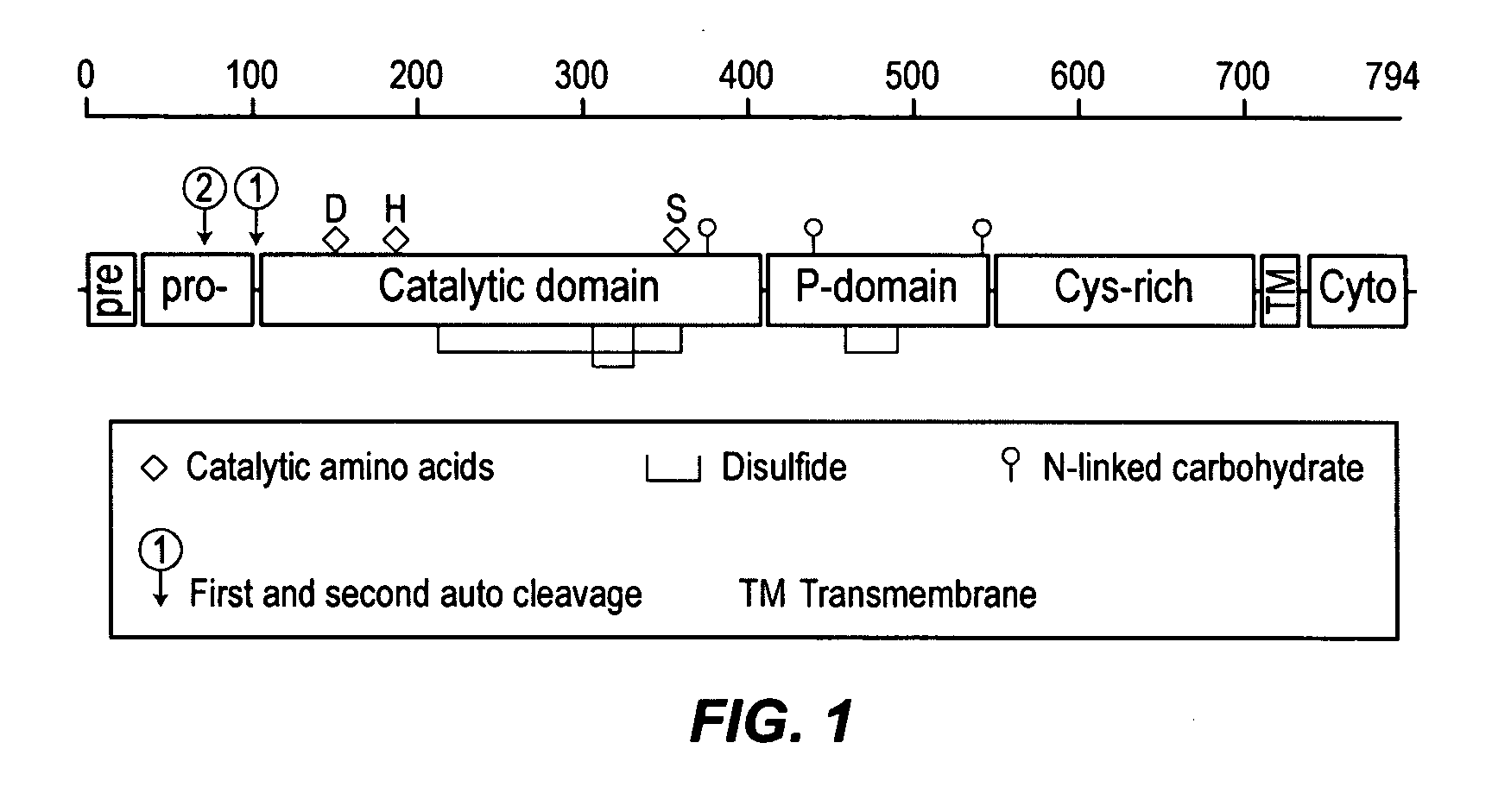
[0090]A detailed description of the furin progenitor plasmids used to construct a rFurin expression plasmid designated #556 is set out in Table 1. Expressed under control of a constitutive cytomegalovirus (CMV) promoter, the mature rFurin contains the catalytic domain, the P domain, and a small portion of the cystine-rich domain whereas regions located C-terminal to amino acid 577 are removed leading to a fully secreted active protease.
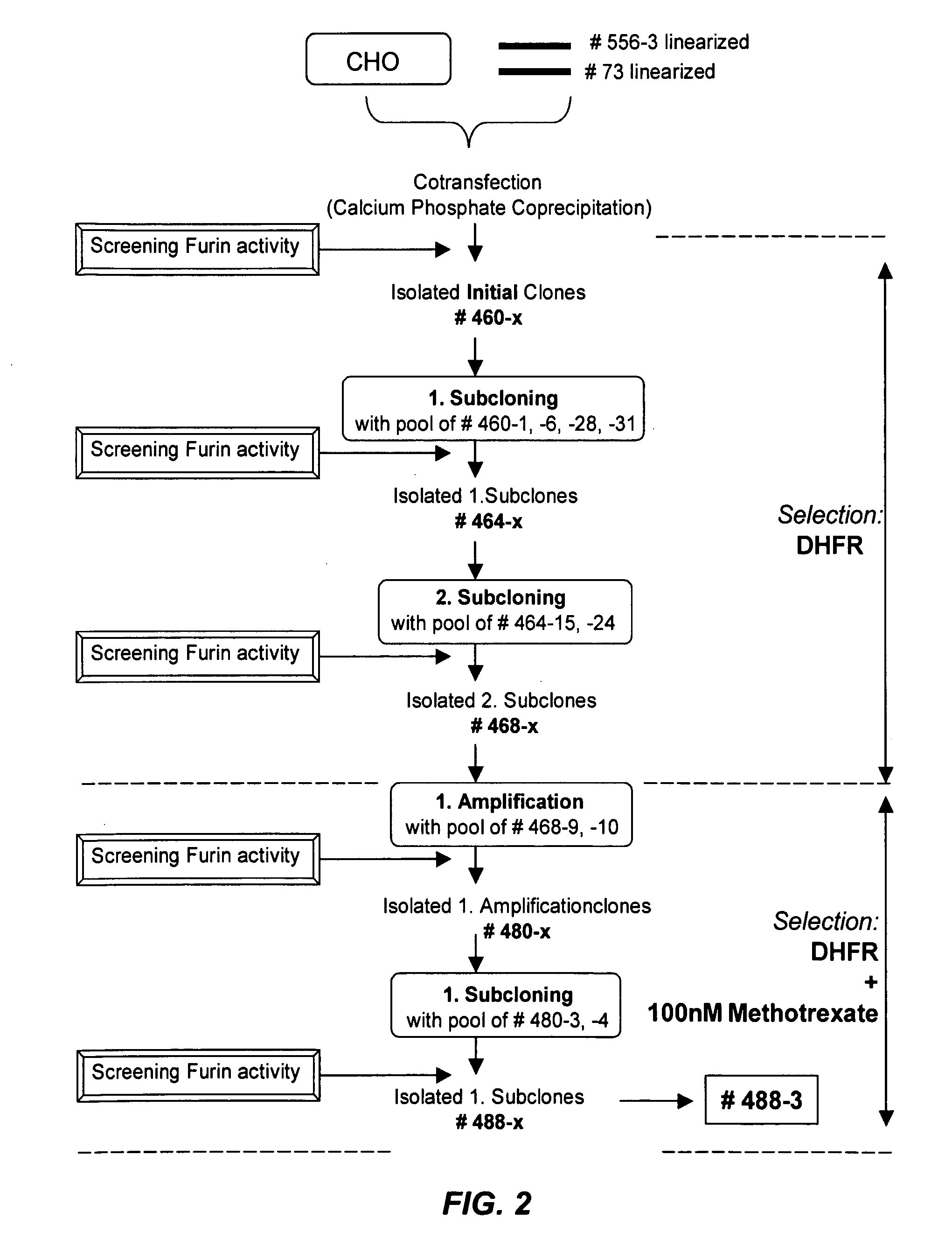
[0091]A description of the construction of the DHFR-vector used as the selection plasmid is depicted in Table 2. For the development of stably expressing CHO / rFurin cell clones designated # 488-3 and # 289-20, CHO cells lacking a functional endogenous DHFR gene were co-transfected with plasmids # 556 and # 73 employing calcium phosphate co-precipitation. Clones secreting high levels of rFurin were selected in several rounds of subcloning and amplification using the DHFR / M...
example 2
Adapting the Recombinant Furin Expressing Cell Clones to Growth in Serum-Free Conditions
[0095]The strategy for cell line adaptation and selection is to adapt the cell line to a serum- and protein-free cell line in either gradually in a step-wise dilution or abruptly. The purpose of this study was to find a CHO cell population growing under serum-free conditions, which was stably producing rFurin. The CHO cell clone #488-3 was used as starting material. The rFurin expressing cell clone CHO #488-3 was changed over to serum-free conditions in three parallel conducted adaptations as set out in detail below.
[0096]The serum depletion process started in spinner flasks with use of microcarriers to find a means to hold back cells in the phase of adaptation, since in that phase cells usually show slow growth. By using this method, it was possible to avoided, during subsequent media changes, diluting the cells to such concentrations where growth could be inhibited.
[0097]Three variants of an in...
example 3
Optimization for Manufacturing Recombinant Furin in Animal Protein-Free Medium
[0132]This example describes the development and optimization process for the culture of the rFurin expressing CHO clone #488-3. Specific medium optimization with regard to amino acids, glucose, and NaHCO3 concentration was carried out, which resulted in increased cell growth rates and higher productivities of the fermentation process. Optimization for inline controlled process parameters was carried out with the optimized medium formulation for pO2 (10%, 20% and 50%), and a factorial experiment was carried out to determine optimum pH (range 7.1-7.3) and temperature (range 35.1° C.-37.9° C.), which resulted in a significant yield improvement for CHO clone #488-3 when fermentation was carried out at lower temperatures between 35°-36° C.
[0133]As possible production modes, chemostat cultures and batch reefed cultures were compared, indicating that both process types are suitable for the manufacturing of rFuri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com