Devices and methods for delivery of a therapeutic agent through a pneumostoma
a technology of pneumostoma and therapeutic agent, which is applied in the direction of respirator, catheter, wound drain, etc., can solve the problems of increased exhalation work, significant underdiagnosis of psoriasis, and hyperinflation of the lung, and achieve the effect of stabilizing the artificial apertur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0074]The following description is of the best modes presently contemplated for practicing various embodiments of the present invention. The description is not to be taken in a limiting sense but is made merely for the purpose of describing the general principles of the invention. The scope of the invention should be ascertained with reference to the claims. In the description of the invention that follows, like numerals or reference designators will be used to refer to like parts or elements throughout. In addition, the first digit of a reference number identifies the drawing in which the reference number first appears.
Pneumostoma Formation and Anatomy
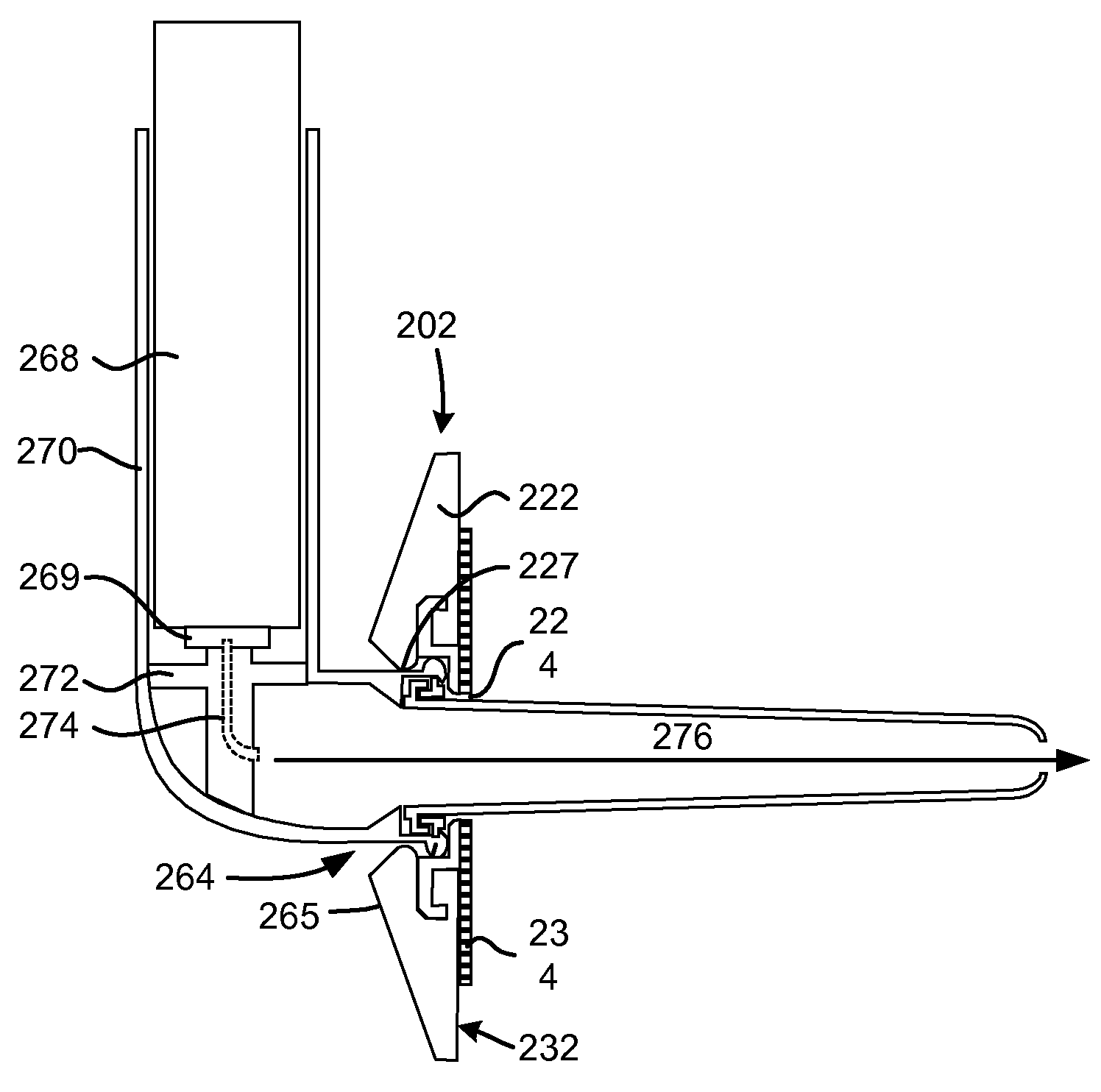
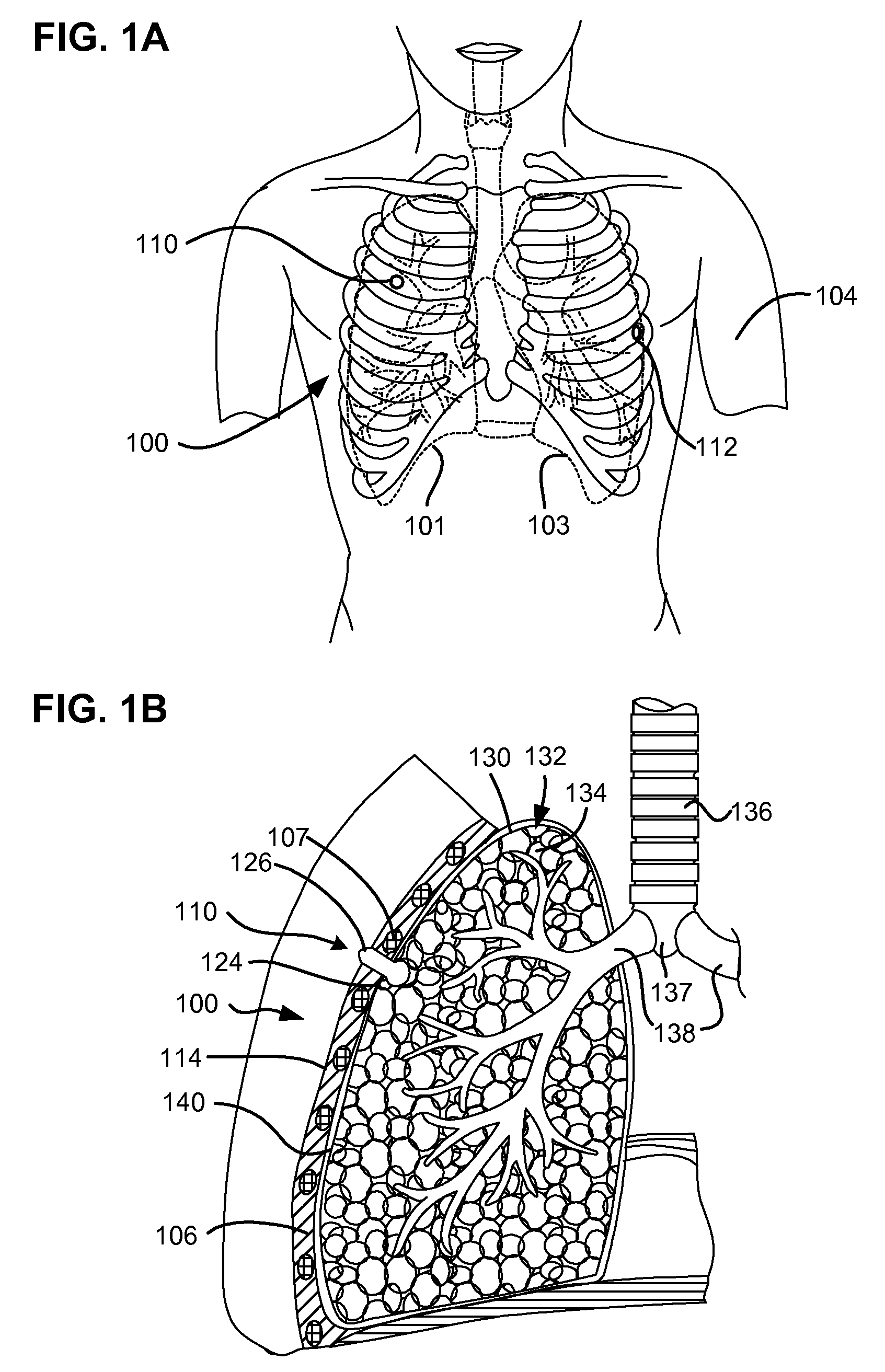
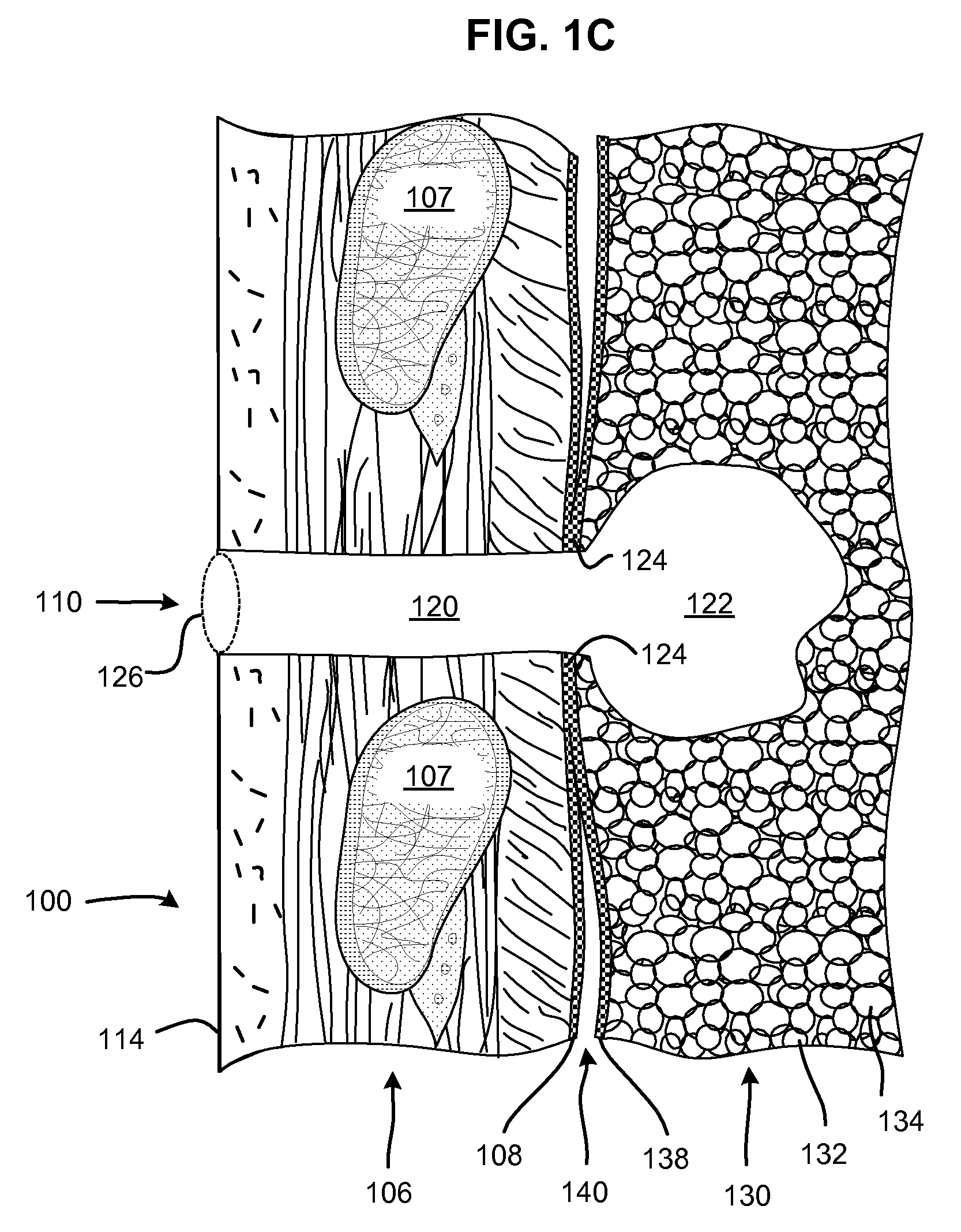
[0075]FIG. 1A shows the chest of a patient identifying alternative locations for creating a pneumostoma that may be managed using the system of the present invention. A first pneumostoma 110 is shown on the front of the chest 100 over the right lung 101 (shown in dashed lines). The pneumostoma is preferably positioned over the third i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com