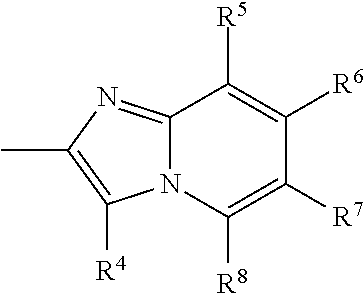
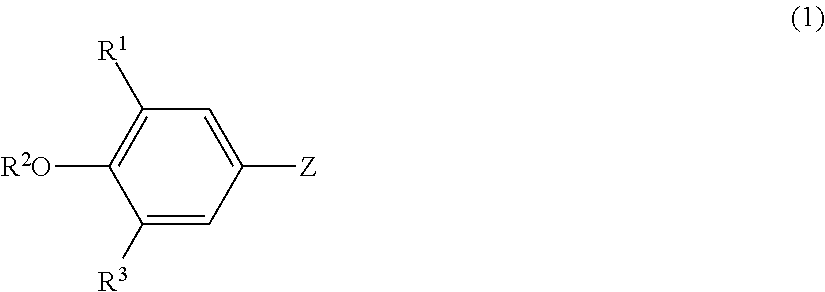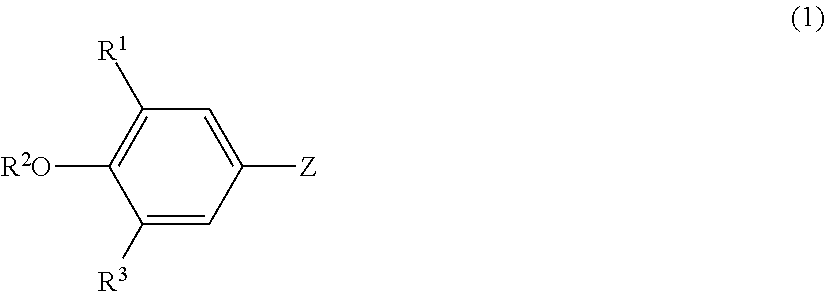Lipoprotein lipase-activating compositions comprising benzene derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-(4-benzyloxy-3-methoxyphenyl)imidazo[1,2-a]pyridine
(Step 1)
[0095]At a temperature of 0° C., 28.5 g (75.8 mmol) of phenyltrimethylammonium tribromide was added over 75 minutes to 120 ml of an anhydrous tetrahydrofuran solution of 12.0 g (72.2 mmol) of 4′-hydroxy-3′-methoxyacetophenone. This mixture was stirred for 2 hours at 0° C. and 30 minutes at room temperature. The reaction suspension thus obtained was concentrated under reduced pressure, mixed with 100 ml of ethyl acetate / hexane (1:1 v / v), and stirred for 30 minutes at 0° C. The crystalline phenyltrimethylammonium tribromide present in the suspension was removed by suction filtration and rinsed with 50 ml of ethyl acetate / hexane (1:1 v / v). The filtered solution was concentrated under reduced pressure, thereby giving 30 g of crude product.
[0096]At room temperature, 14.95 g (158.9 mmol) of 2-aminopyridine was added to 150 ml of an acetonitrile solution of the crude product (30 g) obtained above. The mixture was s...
example 12
Preparation of 3-(4-benzyloxy-3-methoxyphenyl)imidazo[1,2-a]pyridine
(Step 1)
[0100]To a solution of 4.23 g (16.9 mmol) of 2-methoxy-4-(4,4,5,5)-tetramethyl-1,3,2-dioxaborane-2-yl phenol in 200 ml dry DMF were added 5.0 g (25.4 mmol) of 3-bromoimidazo[1,2-a]pyridine, 0.39 g (0.34 mmol) of tetrakis(triphenylphosphinato)palladium [0] (Pd(PPh3)4 wherein Ph is phenyl), and 42.25 ml of 2M aqueous potassium phosphate solution. The mixture was stirred for 20 hours at 80° C. After reaction, DMF was distilled off under reduced pressure, and the residue was purified using a silica gel column (eluant: methanol / methylene chloride=2 / 98 to 4 / 96).
[0101]The crystals thus obtained were recrystallized using methanol-hexane, thereby producing 2.82 g of 3-(4-hydroxy-3-methoxyphenyl)imidazo[1,2-a]pyridine (yield: 70%). This compound will be referred to as “Example Compound 131”.
(Step 2)
[0102]To 1 ml of DMF were added 24 mg (0.1 mmol) of the compound obtained in Step 1 (Example Compound 131), 65 mg (0.2 mm...
example 23
Preparation of 6-[4-(4-chlorobenzyloxy)-3-methoxyphenyl]imidazo[2,1-b]thiazole
(Step 1)
[0104]To 600 ml of an anhydrous THF solution of 100 g 4′-hydroxy-3′-methoxyacetophenone was added 237.5 g phenyltrimethylammonium tribromide over 3 hours at a temperature of 0° C., followed by stirring at 0° C. for 6 hours and at room temperature for 13 hours. The reaction solution thus obtained was concentrated under reduced pressure, mixed with 500 ml of ethyl acetate, and stirred at 0° C. for 1 hour. The precipitated crystals were removed by suction filtration, and the filtered solution was concentrated under reduced pressure, thereby yielding 265 g of oily matter.
[0105]This oily matter was dissolved in 400 ml of anhydrous DMF, and the solution thus obtained was added to 60 g of 2-aminothiazole followed by stirring at room temperature for 30 minutes and 40° C. for 3.5 hours. The reaction mixture thus prepared was diluted with 400 ml of ethyl acetate and left for 15 hours at room temperature. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com