Detecting Cell Surface Markers
detection method technology, applied in the field of detection and quantification of cell surface markers, can solve the problems of limited statistical confidence, poor long term stability, and not widely used in diagnostic pathology, and achieve the effect of accurately quantifying a cell surface marker in the sampl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0147]Slide Preparations
[0148]A total of 448 consecutive cases were selected for this study of which 425 were successfully stained, reviewed and digitized. All cases were formalin fixed and paraffin embedded and were processed in the routine diagnostic laboratory of the institute of origin according to standardised protocols.
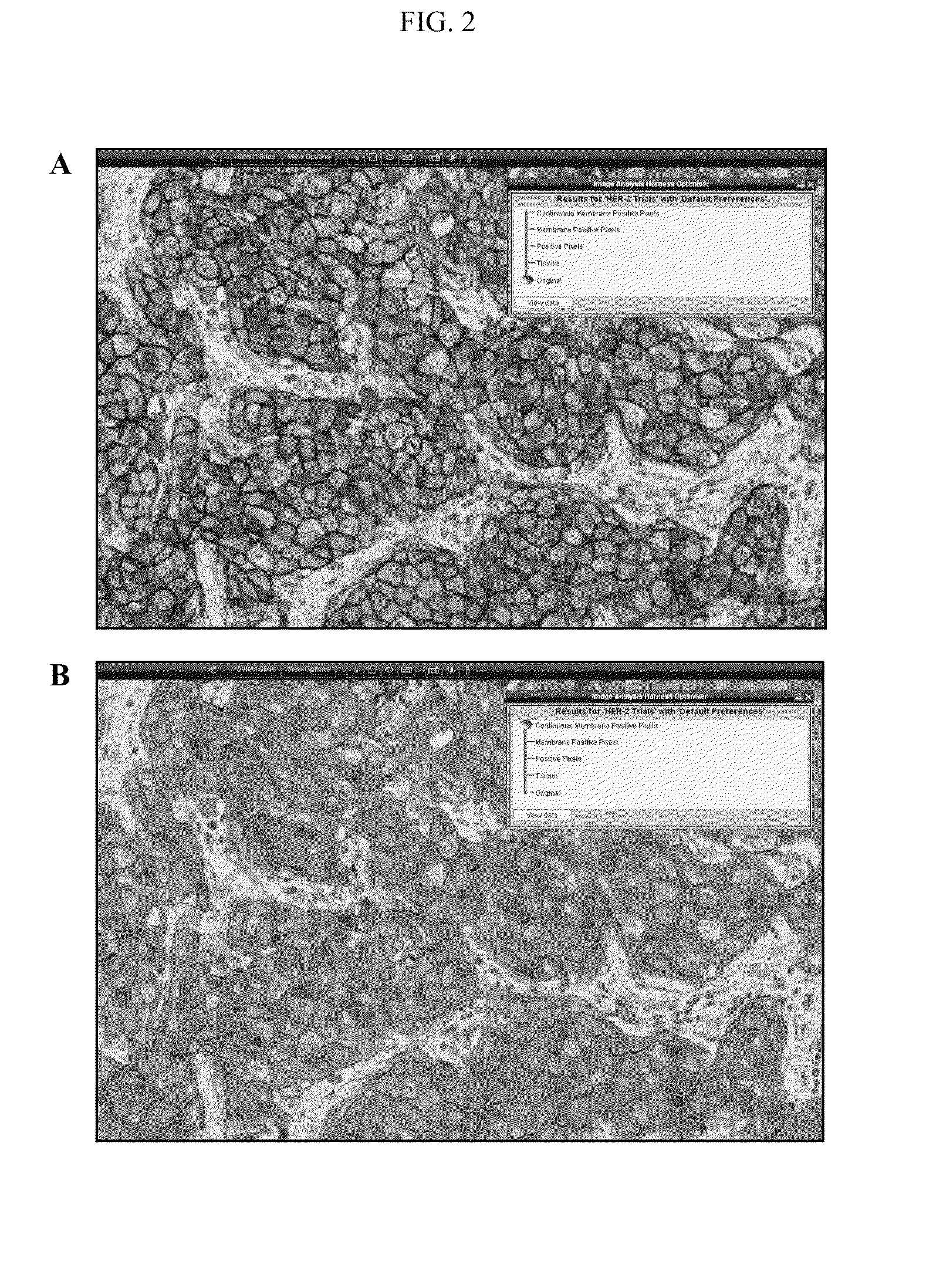
[0149]Immunohistochemistry
[0150]The cases were assessed for HER-2 protein expression using Dako HercepTest® (n=144), Leica Oracle™ HER-2 (n=140) or Ventana Pathway® HER-2 (4b5) (n=141) according to the manufacturer's instructions. In all cases, suitable negative and positive control slides were treated in a similar manner to ensure appropriate staining
[0151]Fluorescent in situ Hybridization
[0152]A representative cohort of cases was selected for FISH testing for verification purposes. Of the 425 cases supplied, 219 were analysed for HER-2 gene amplification using the PathVysion® HER-2 DNA probe kit and paraffin wax pre-treatment kit (Vysis Inc, UK) in the facilit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cell surface | aaaaa | aaaaa |
| threshold value | aaaaa | aaaaa |
| fluorescent in- | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com