Method for predicting a need for renal replacement therapy (RRT)
a technology of renal replacement therapy and prediction method, which is applied in the direction of measuring devices, microbiological testing/measurement, instruments, etc., can solve the problems of dialysis, no drug therapy available to counteract, and patients will require rr
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Use of πGST as a Biomarker for AKI in Patients Undergoing CT Surgery
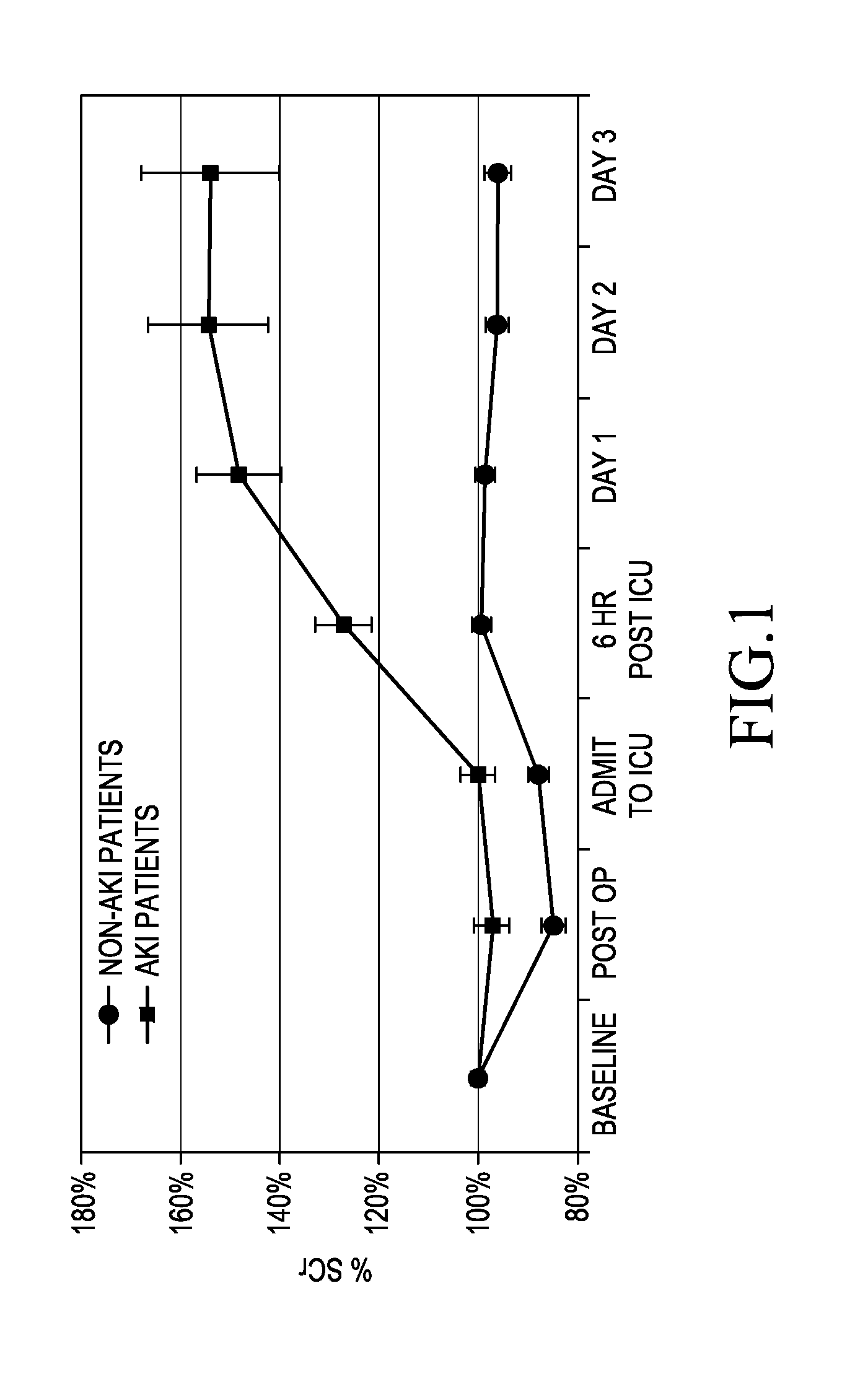
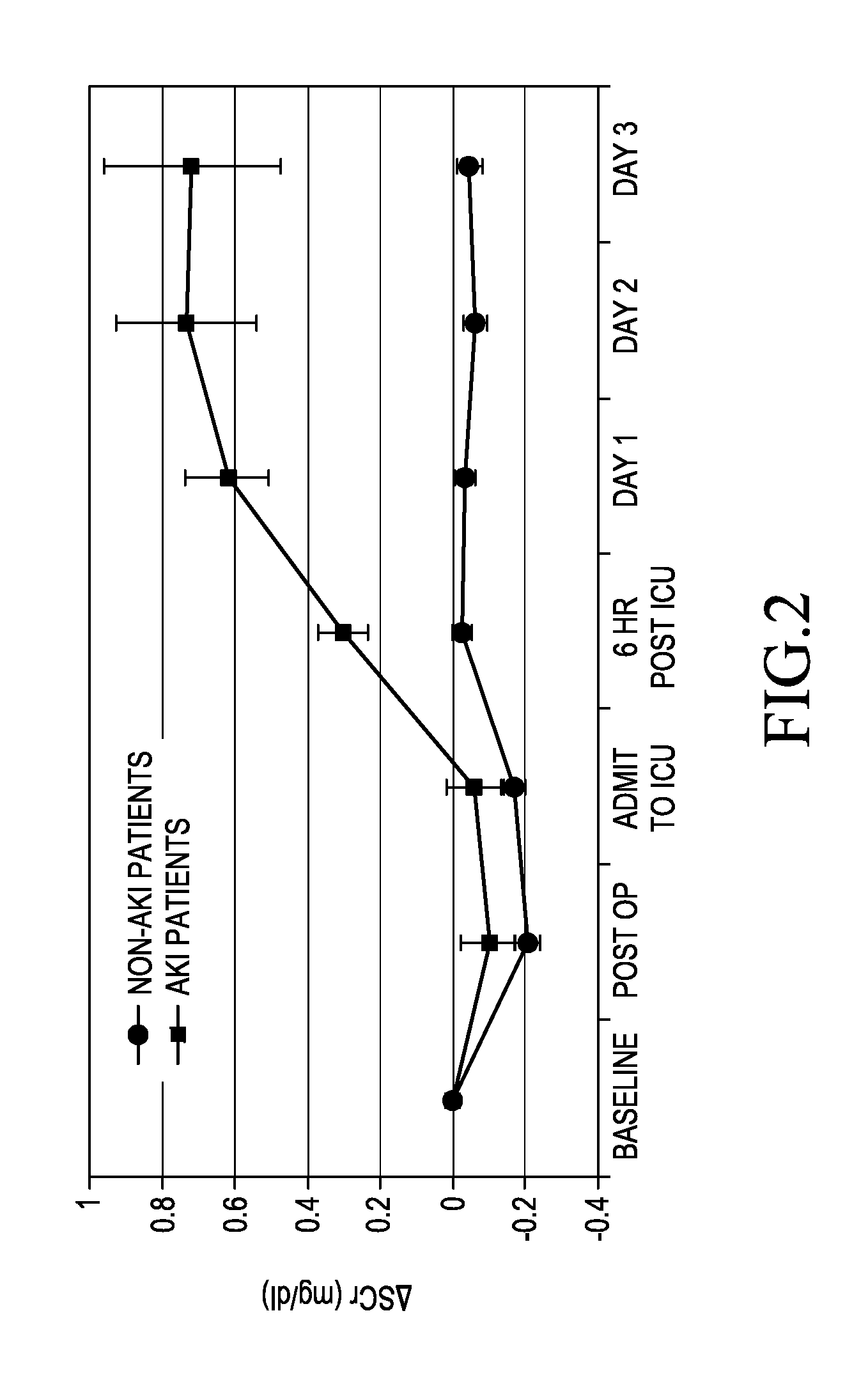
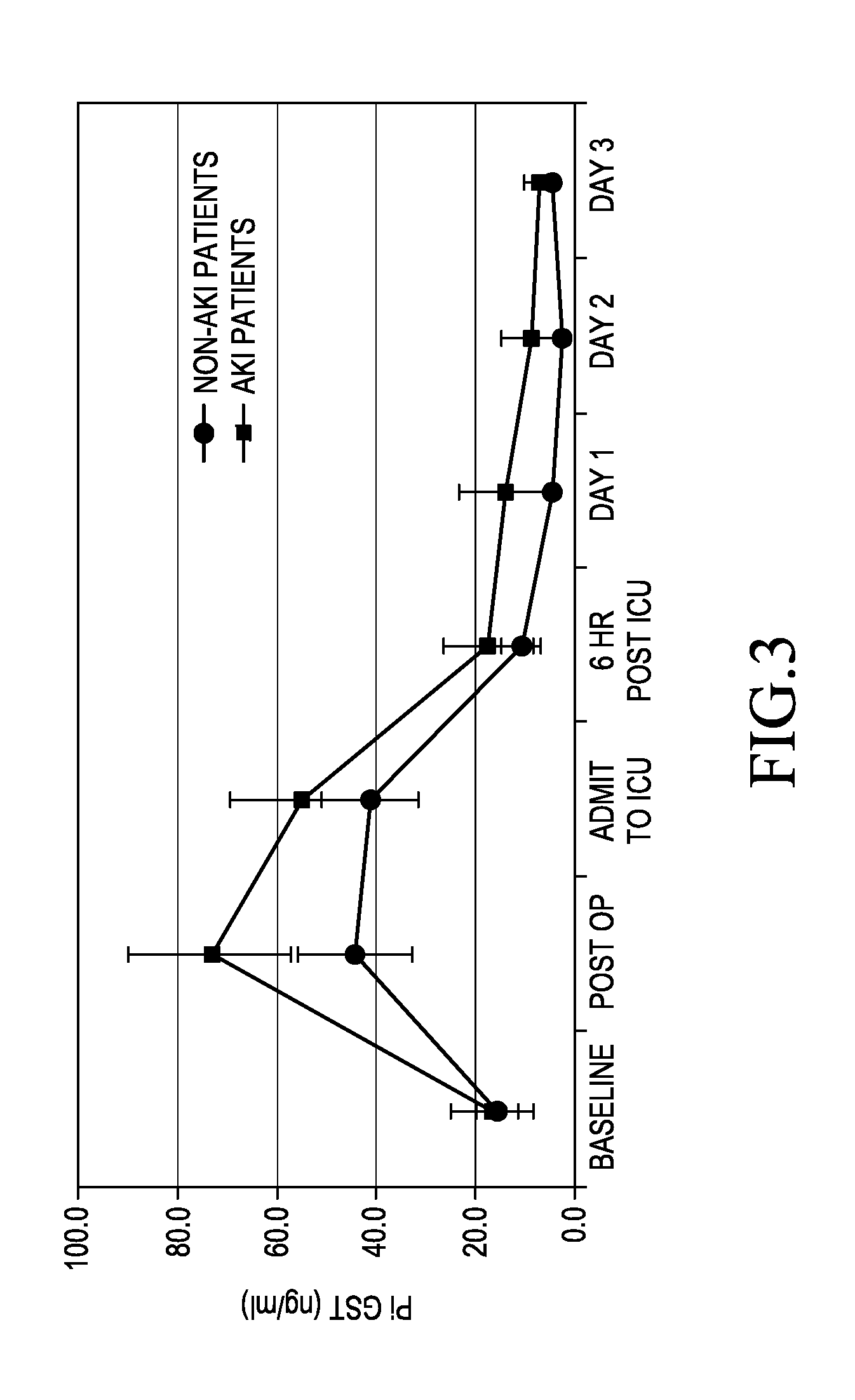
[0046]A retrospective study of 68 patients who had undergone elective CT surgery at the University of Chicago Hospital was carried out.
[0047]The patients were screened and approached for enrolment. The patients were excluded if they met any of the following criteria:
Pre-existing End Stage Renal Disease (ESRD) (on RRT) or Renal Transplant.
[0048]Age <18 years old.
Use of radiocontrast within 24 hours of surgery.
Change in thyroid hormone replacement dose in the last 2 weeks
Change in thyroid chronic corticosteroids dose in the last 2 weeks
Unstable renal function (ΔSerum Creatinine ≧0.2 mg / dl in the last 2 months of Oliguria defined as <400 ml / day).
[0049]Urine and blood samples were collected and stored.
[0050]The urine samples were tested for the presence of πGST using the aforementioned πGST EIA available from Biotrin International Limited (Catalogue Number BIO85).
[0051]Serum Creatinine (SCr) was measured using the Jaffé...
example 2
Use of πGST as a Biomarker for a Requirement for RRT Patients Undergoing CT Surgery
[0063]A study was carried out on the 68 patients, the subject of Example 1, using the same methodology for the detection of SCr and πGST.
[0064]Seven patients out of the 68 patients tested required RRT. The results are shown in Table 2.
TABLE 2BaselineCreatinineHours inCreatinineat RRTICU prior(mg / dL)(mg / dL)to RRTIndication15.035.425.3Refractory Hyperkalemia (6.0), Oliguria21.493.4851.2Anuria, Elevated creatinine, Shock1.36 post-op31.31.4221.6Volume overload, Hypoxia, Oliguria, Hemodynamic instability*AKI not diagnosed using current SCr measures*41.23.7926.8Lactic Acidosis Oliguria, Shock, Elevated creatinine50.991.283Lactic Acidosis Anuria, Shock,*AKI not diagnosed using current SCr measures*61.191.745.3Anuria, Shock (3 pressors), Volume overload. Acidosis71.662.881Volume overload, pulmonary edema. Shock
[0065]The time point at which patients requiring RRT would be first diagnosed is shown in Table 3.
TA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com