Compositions containing platelet contents
a technology of platelet contents and compositions, which is applied in the field of compositions containing platelet contents, can solve the problems of not being able to generate primary tumor cultures with high efficiency, not being able to rapidly grow cultures for many applications, and not being able to meet the requirements of a large number of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Source of Platelets
[0078]All donors donating apheresis platelets fulfilled eligibility criteria as defined by AABB Standards for Blood Banks and Transfusion Service and the Food and Drug Administration. Donors were screened using the Uniform Donor History Questionnaire (UDQ) and accompanying educational materials. This questionnaire is a screening document created by a coalition of regulatory, accrediting, and blood collecting institutions consisting of the Food and Drug Administration, Centers for Disease Control and Prevention, Armed Services Blood Program, National Heart Lung and Blood Institute, American Blood Resources Association, AABB, American Red Cross, and America's Blood Centers. Information concerning the UDQ can be found on the World Wide Web at “fda.gov / cber / dhq / dhq.htm.”
[0079]All apheresis platelet donations were tested with the following infectious disease tests: (1) Serologic test for syphilis; (2) HCV EIA-hepatitis C virus antibody test, (3) HCV NAT-hepatitis C vir...
example 2
Preparing Platelet Lysate from Apheresis Platelets
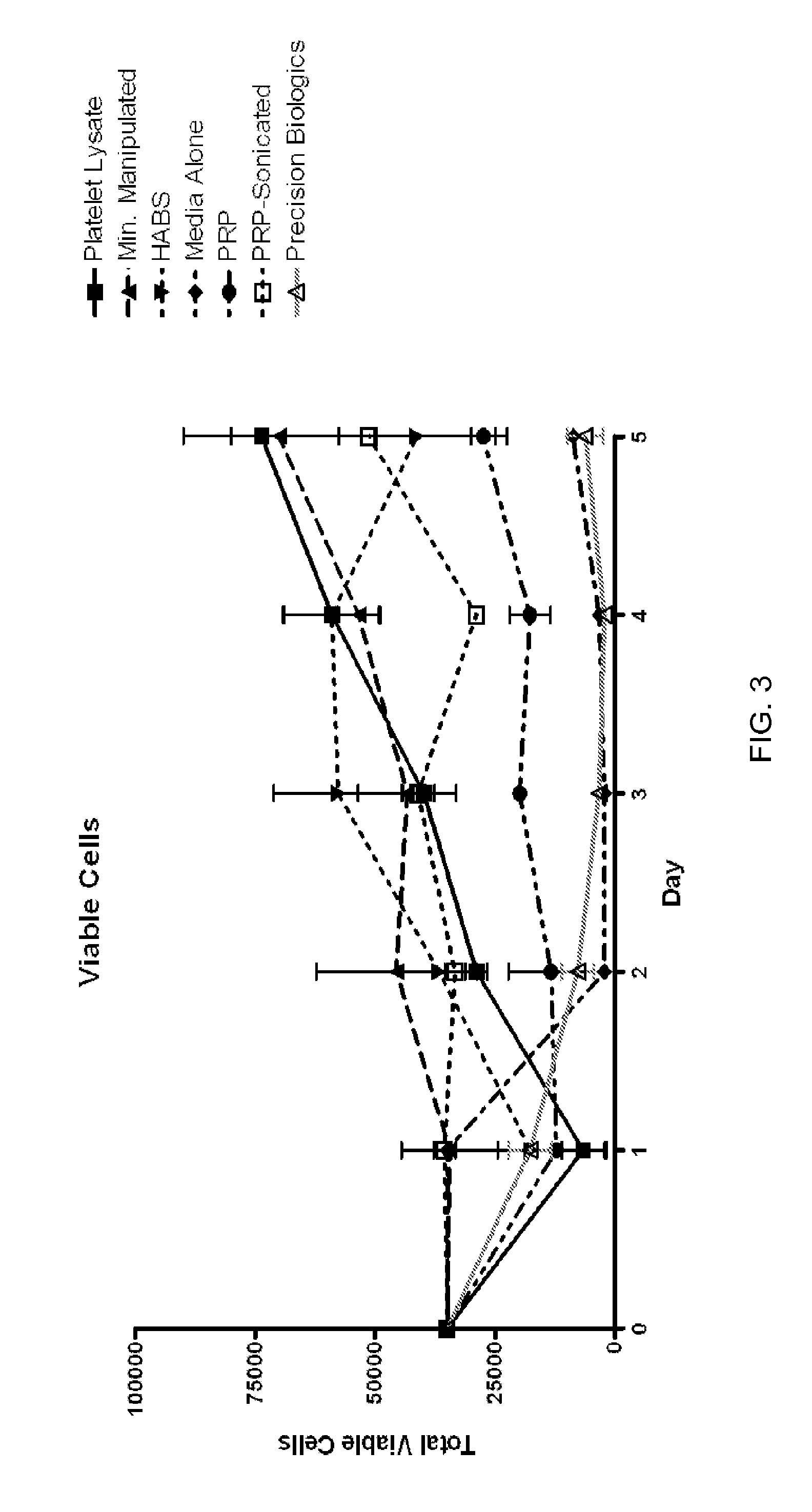
[0082]Apheresis platelets were obtained as described in Example 1. The apheresis platelets used were no more than four days past expiration. A single lot of platelet lysate consisted of ten individual apheresis platelet units, and one lot was used at a time to create a platelet lysate product. The processing for clinical grade reagents can be performed in a clean room suite. Ten individual apheresis platelet units were frozen at −70° C. or colder. After being frozen for at least 24 hours, the units were removed from the freezer and allowed to thaw. The units were thawed at room temperature or at refrigerated temperatures. After thawing was complete, each unit was mixed by massaging the bag. Each thawed platelet bag was placed flat (to minimize breakage of tubing) in a supercold freezer (−70° C. or colder) for a second freeze. After the apheresis platelet units were frozen for at least 24 hours for a second freeze, they were removed f...
example 3
Culturing Cells with Platelet Lysates
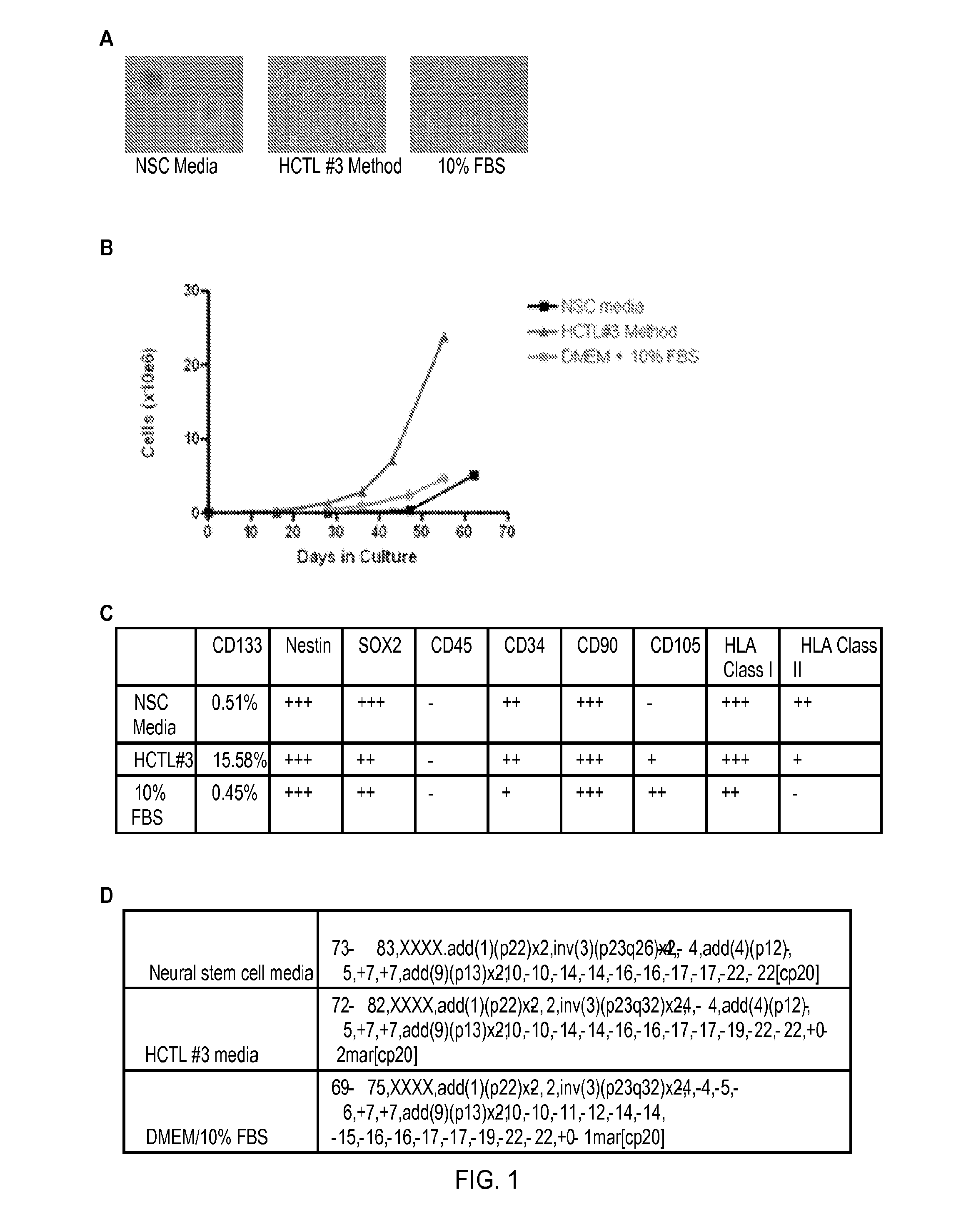
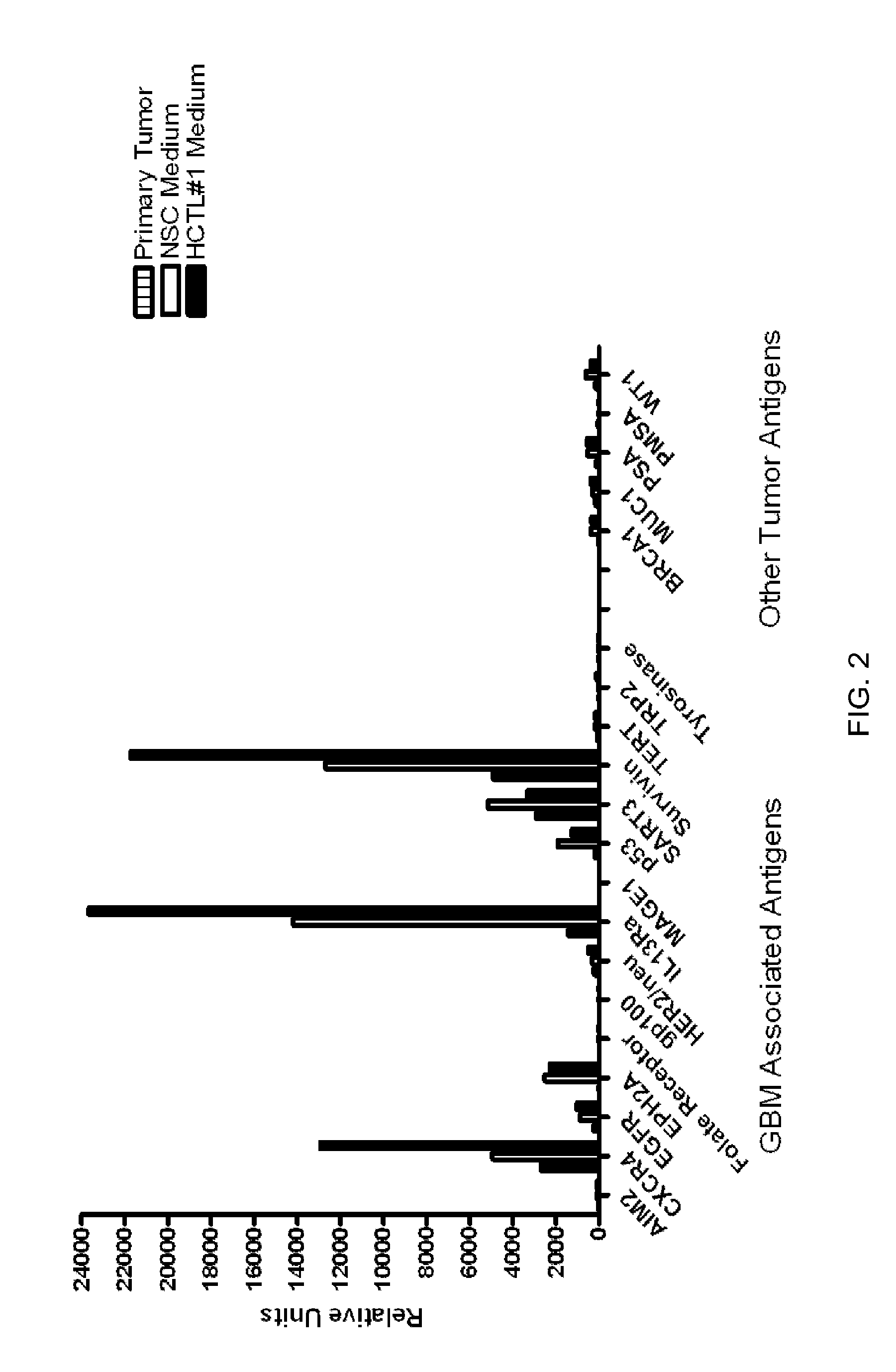
[0092]The phenotypic characteristics of GBM cells cultured with media containing platelet lysates were compared to those of GBM cells cultured using NSC media (Neurobasal media (Invitrogen, Grand Island N.Y.); recombinant EGF and FGF (R&D Systems, Minneapolis, Minn.), N2 and B27 supplements (Invitrogen, Grand Island N.Y.), glutamine and penicillin / streptomycin) or DMEM containing 10% FBS. The media containing platelet lysate (HCTL#3) consisted of Neurobasal media supplement with 5% platelet lysate, glutamine, and penicillin / streptomycin. Cells grown in NSC media formed classical neurospheres enriched in tumor stem cells (FIG. 1A). Cells grown in DMEM 10% FBS became adherent and were usually differentiated (FIG. 1A). Cells grown in HCTL#3 resulted in a mixed population of free-floating neurospheres and adherent neuropheres with superior growth kinetics (FIG. 1A and 1B). Cells cultured in HCTL#3 also exhibited many aspects of neural tumor stem cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com