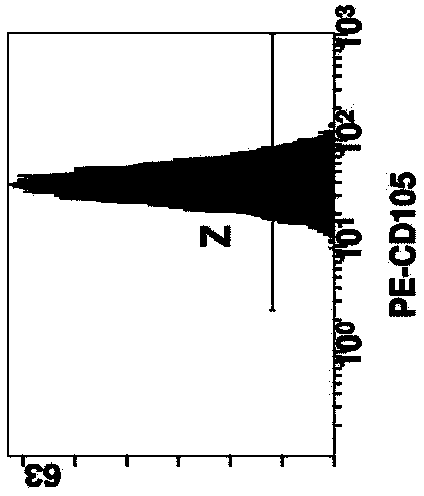
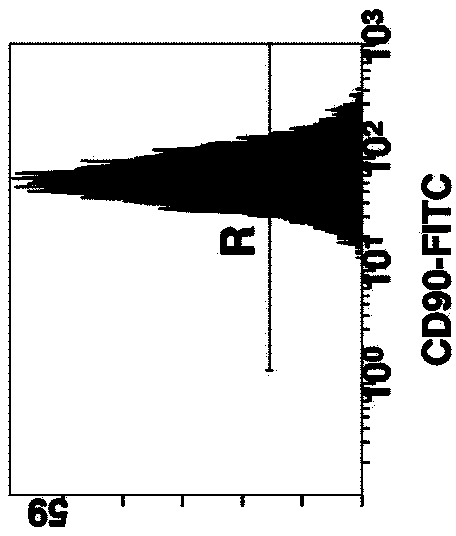
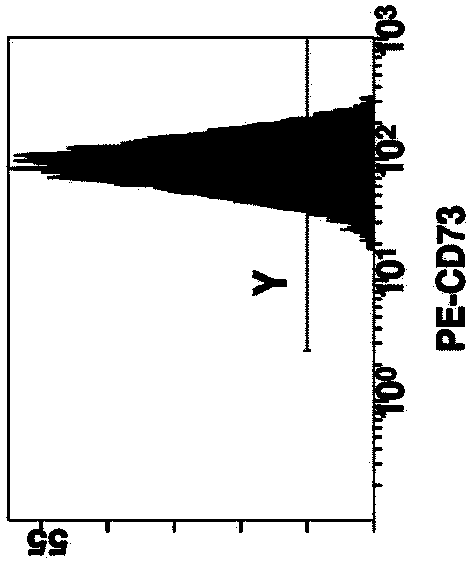
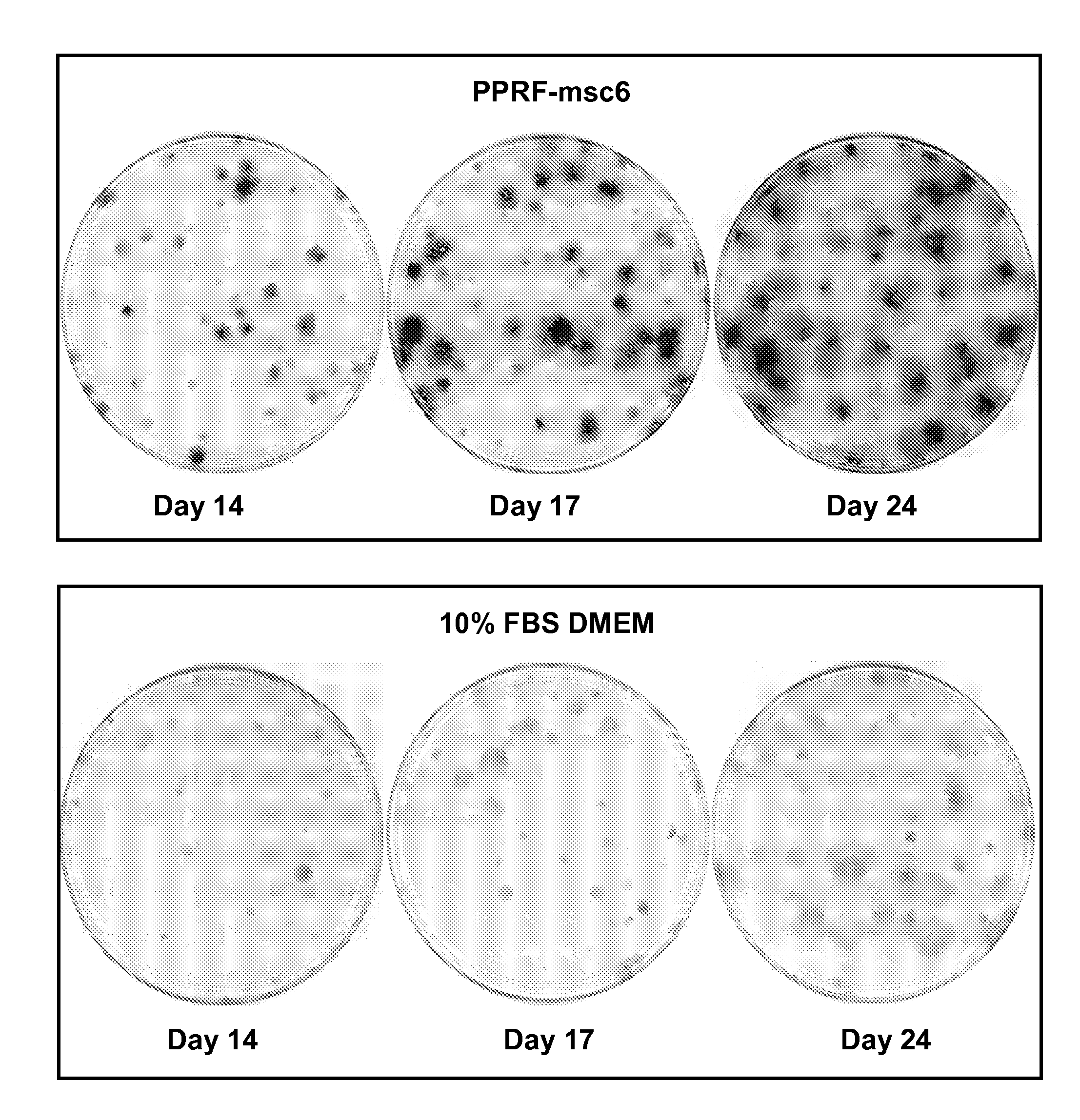
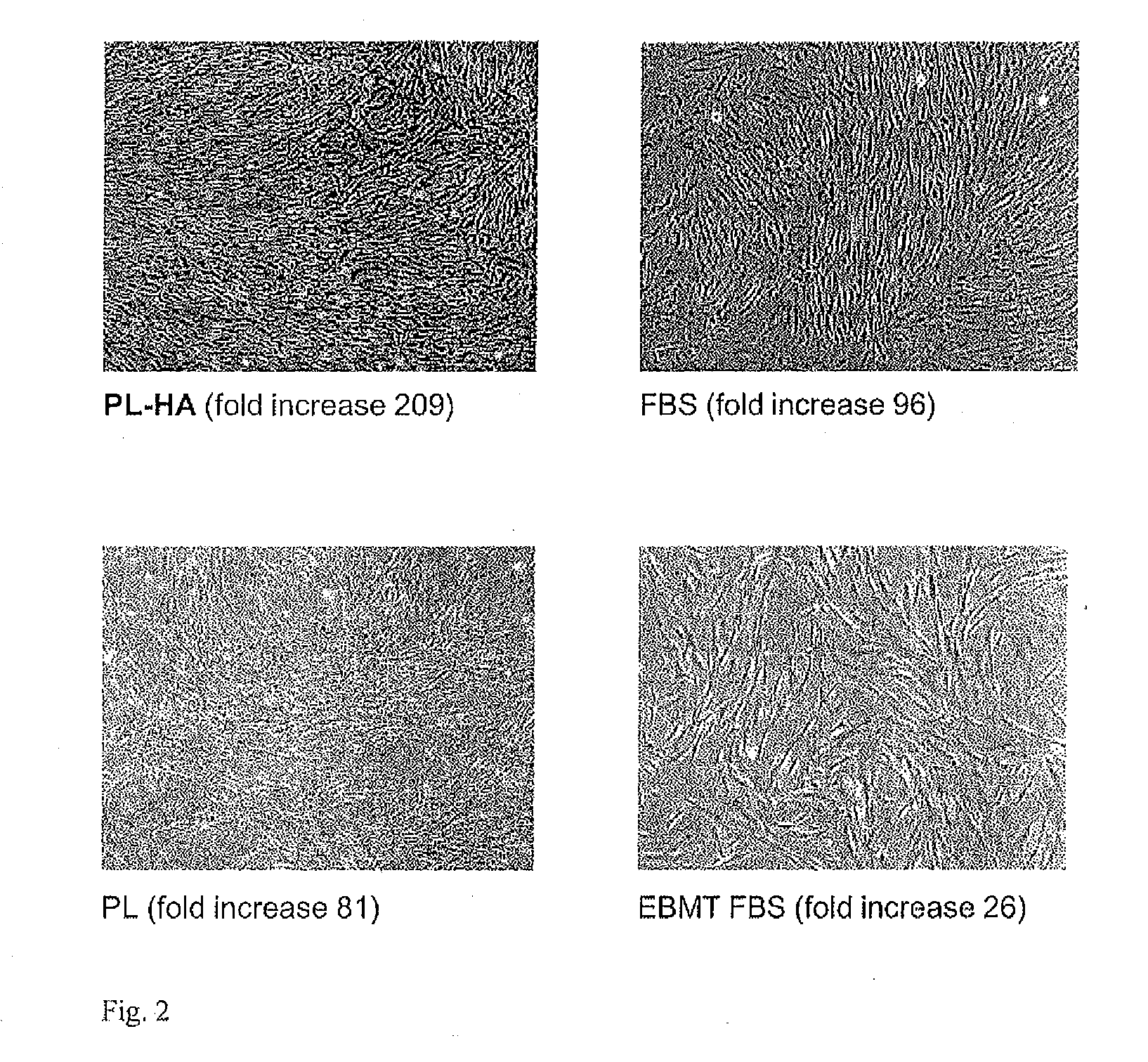
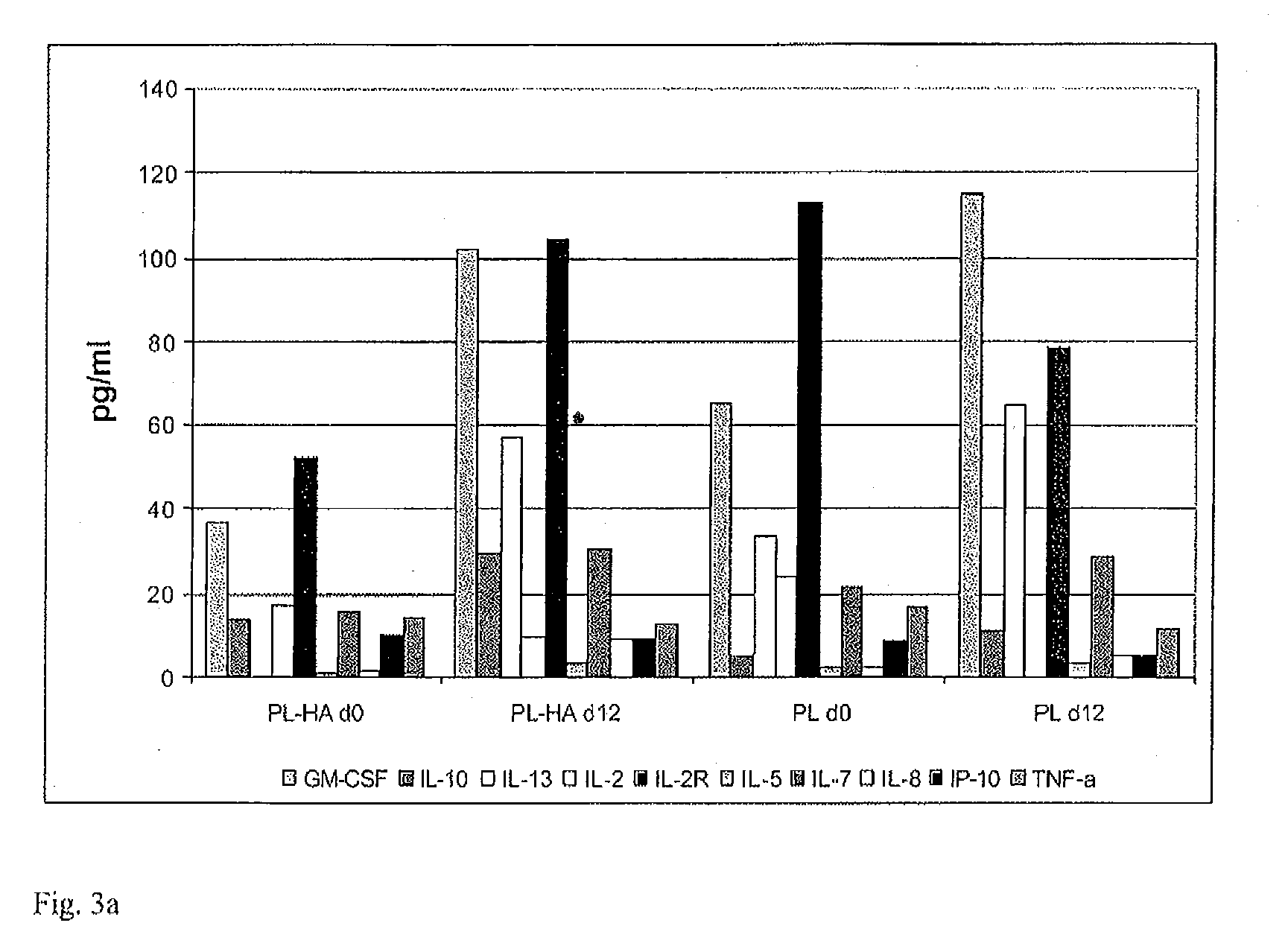
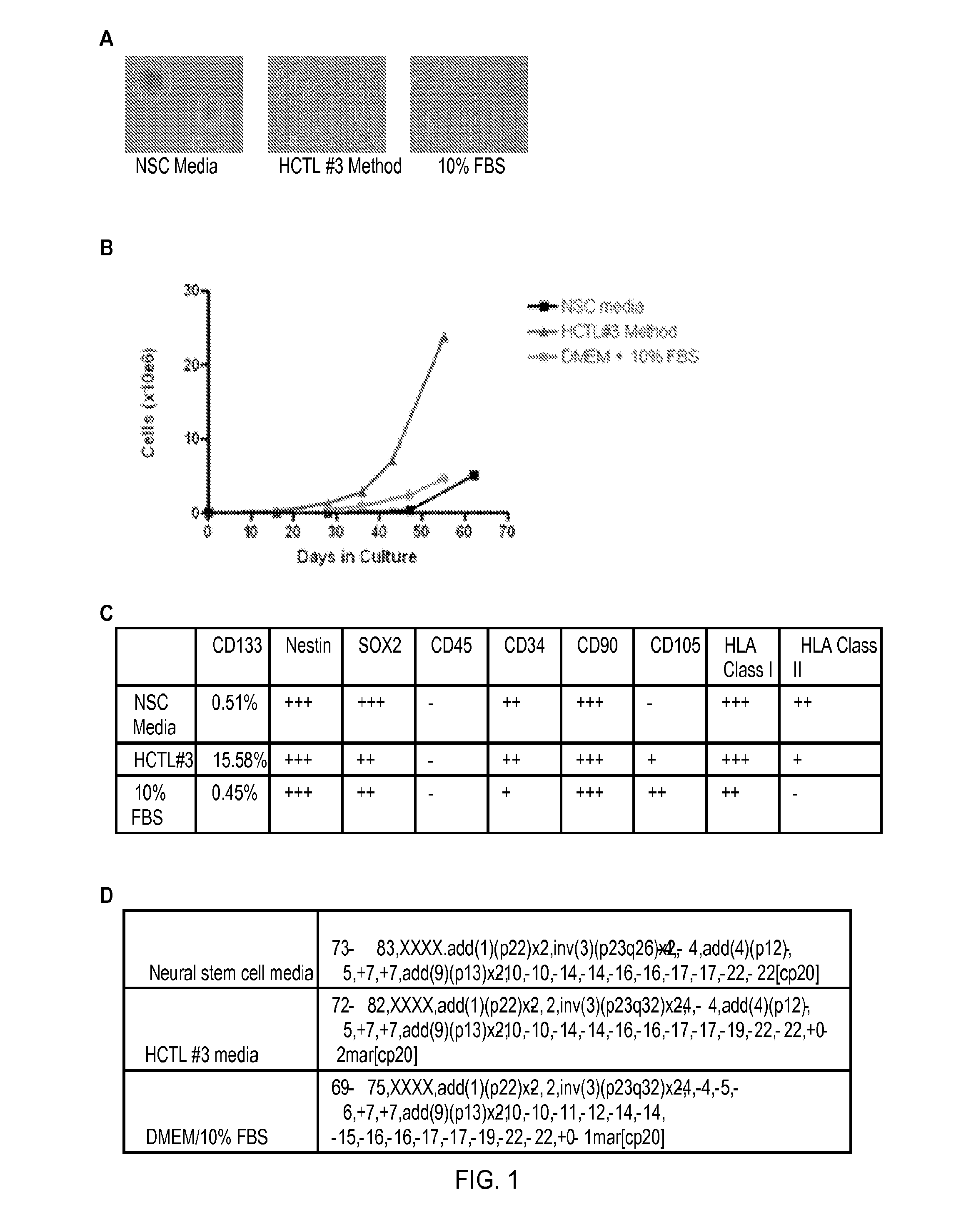
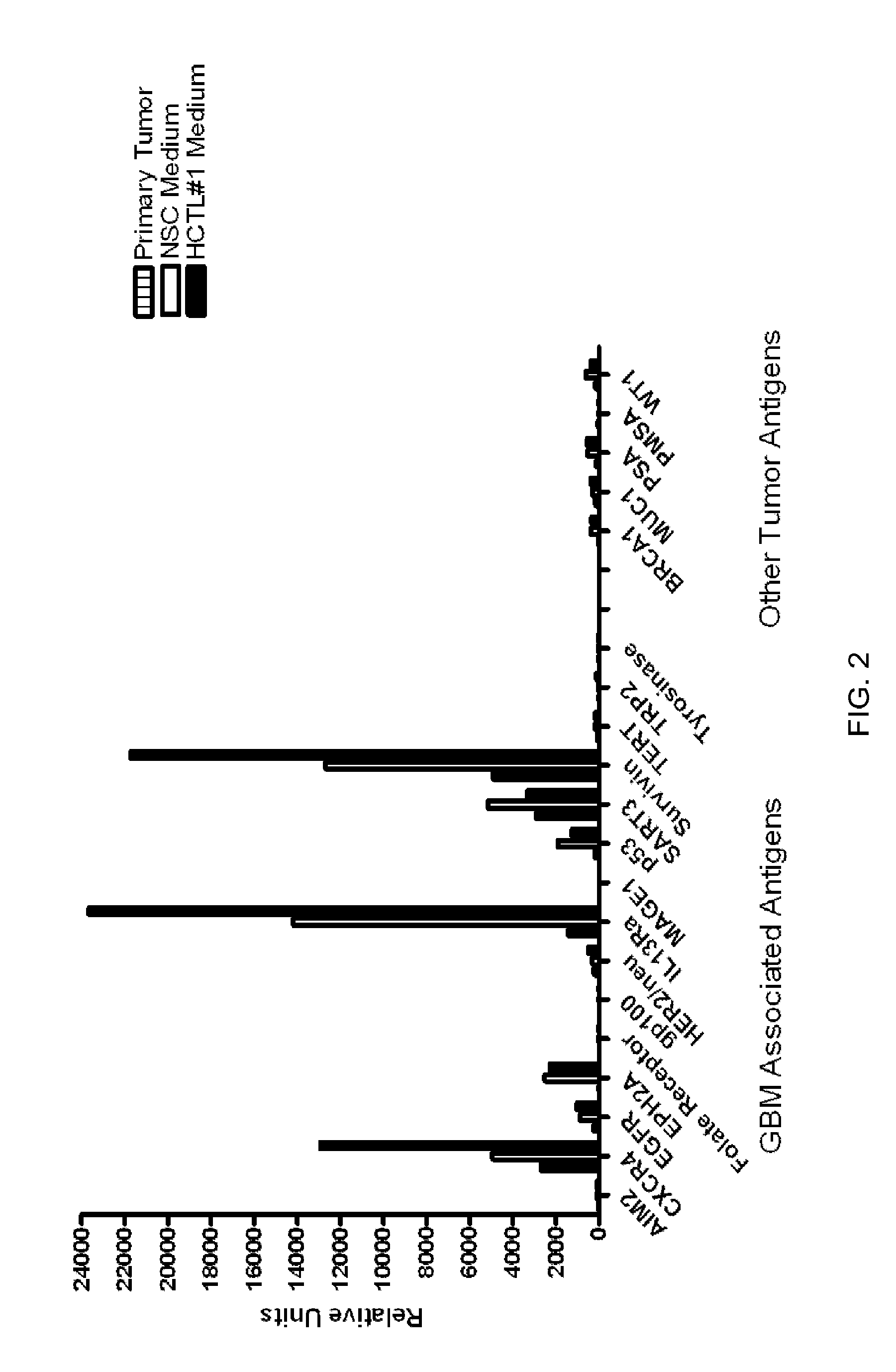
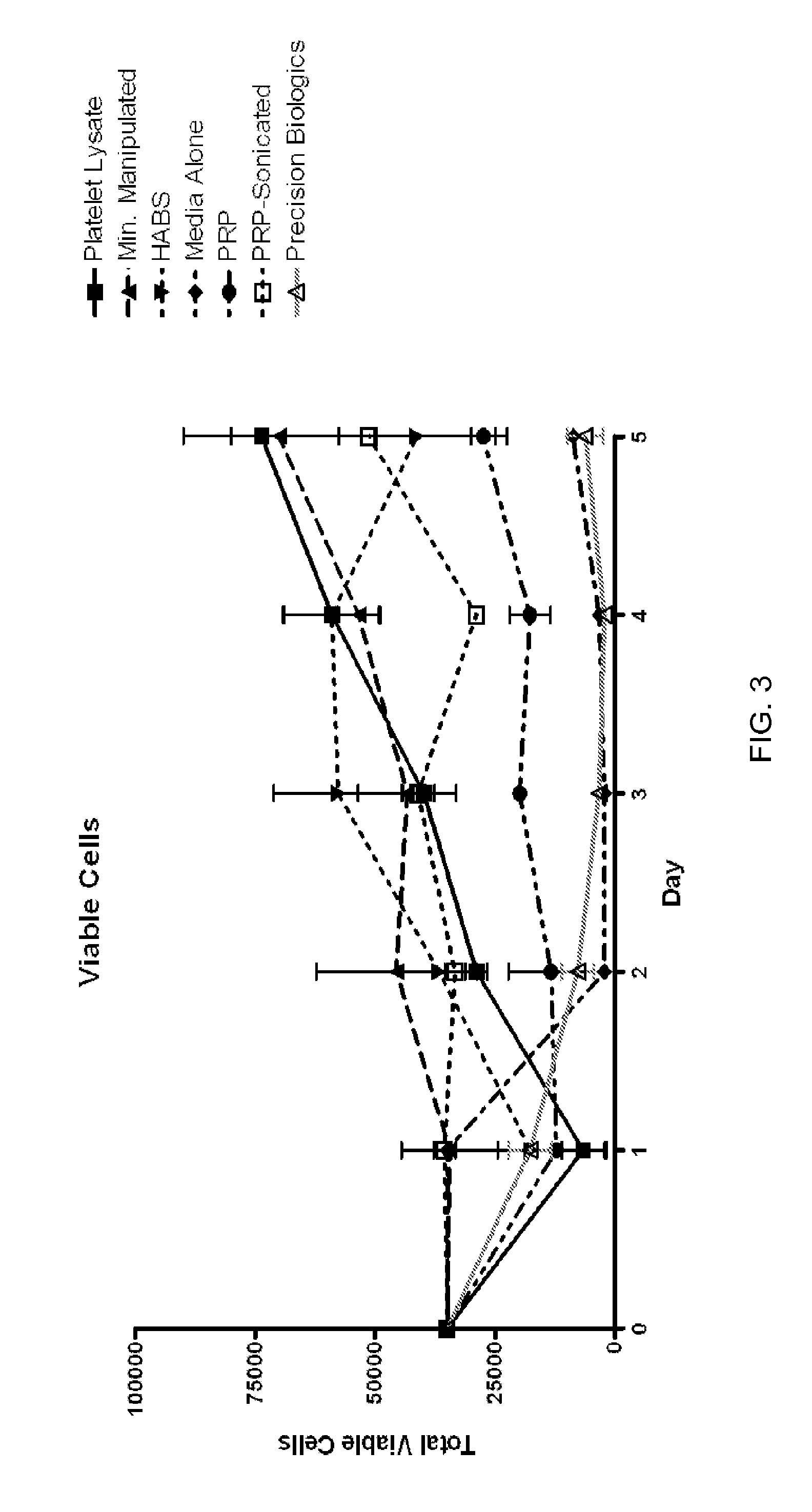
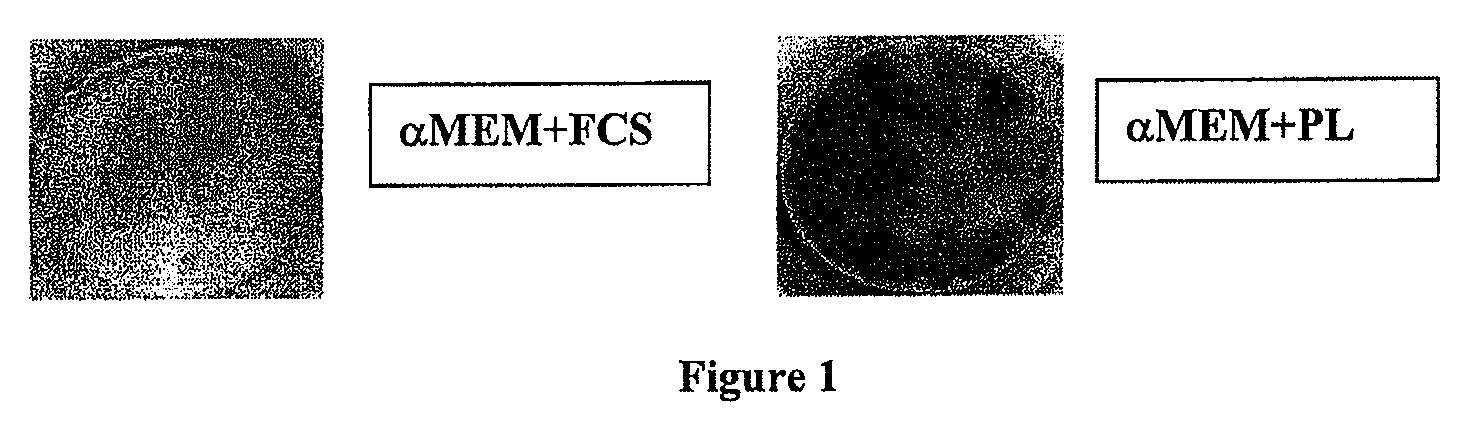
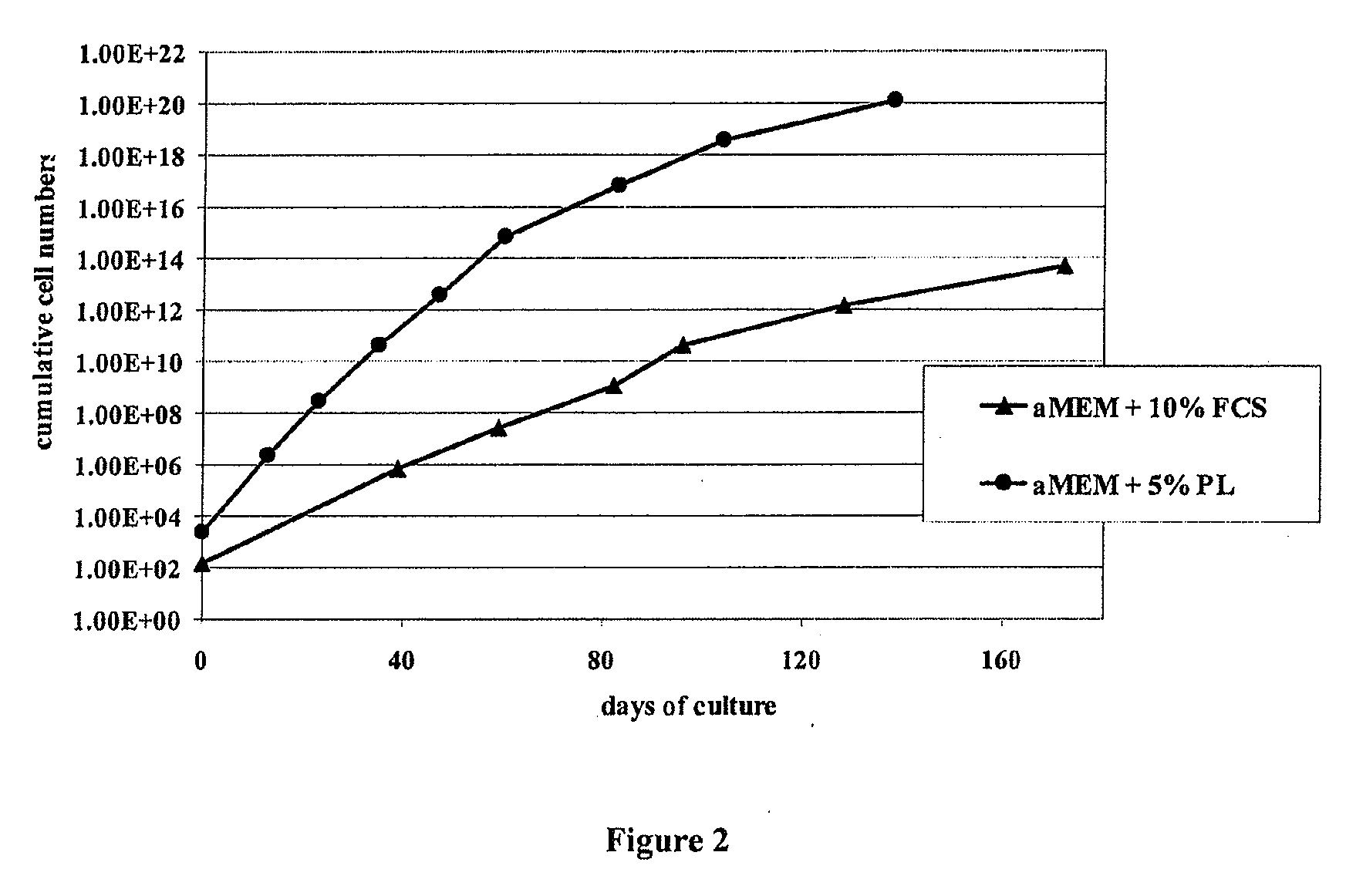
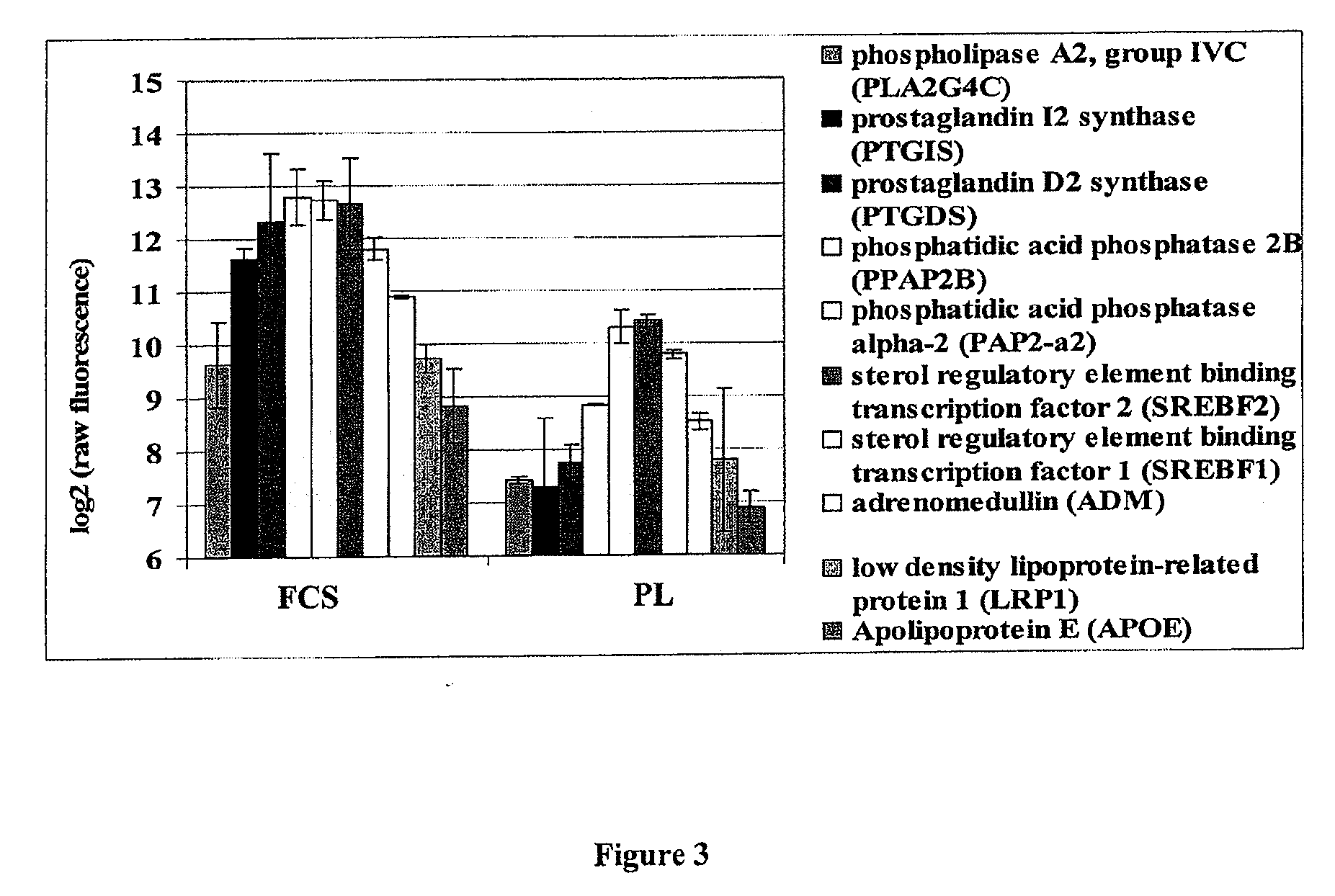
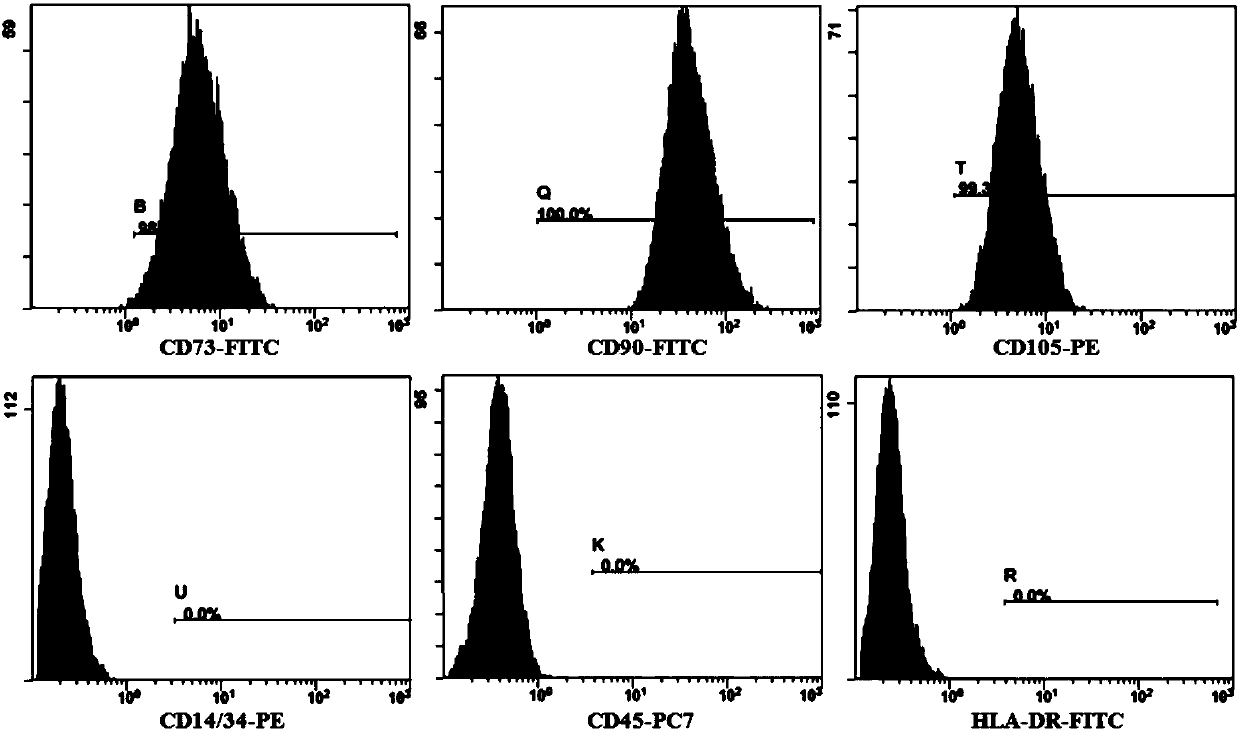
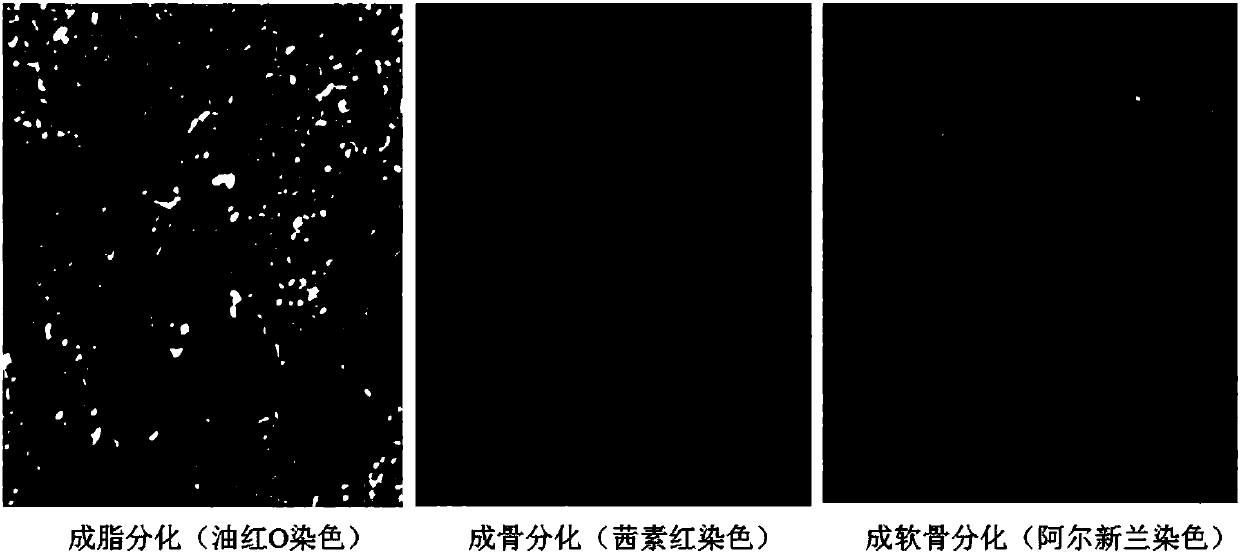
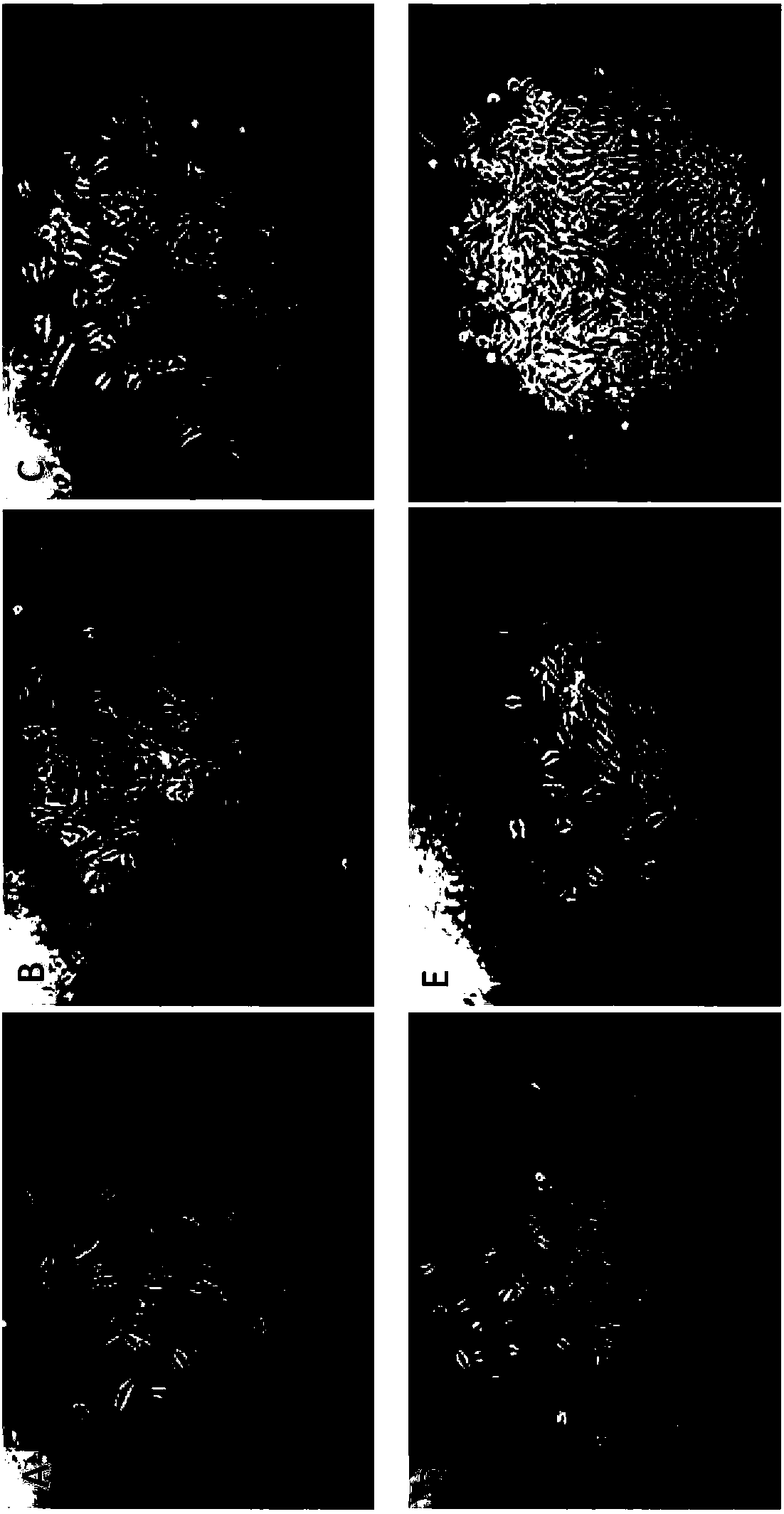
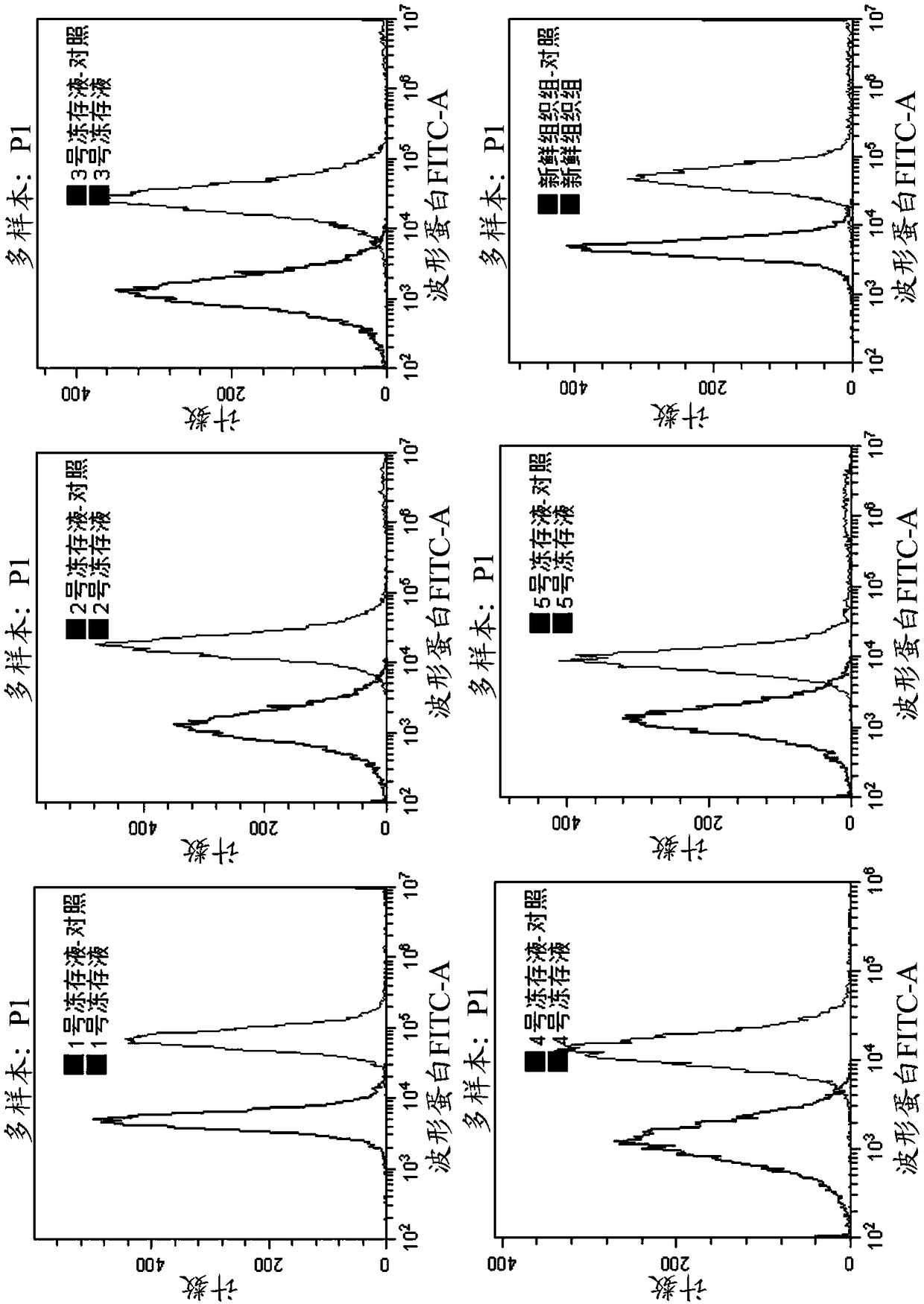
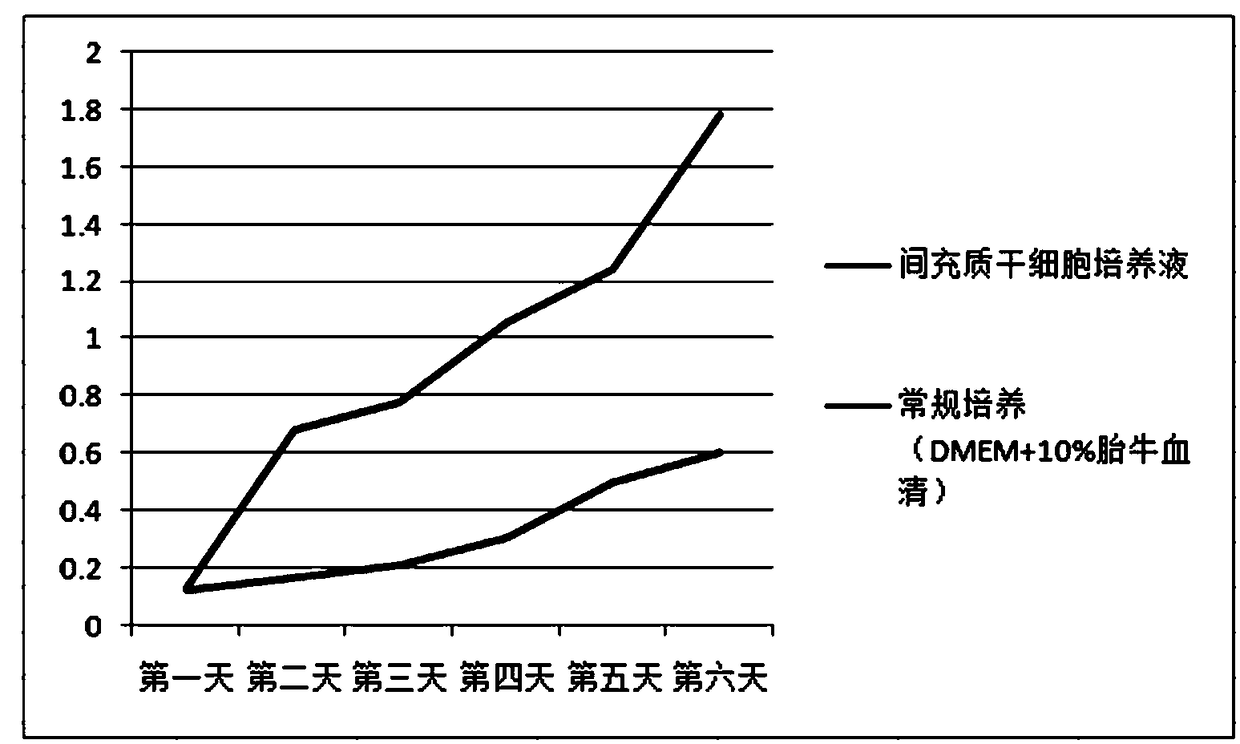
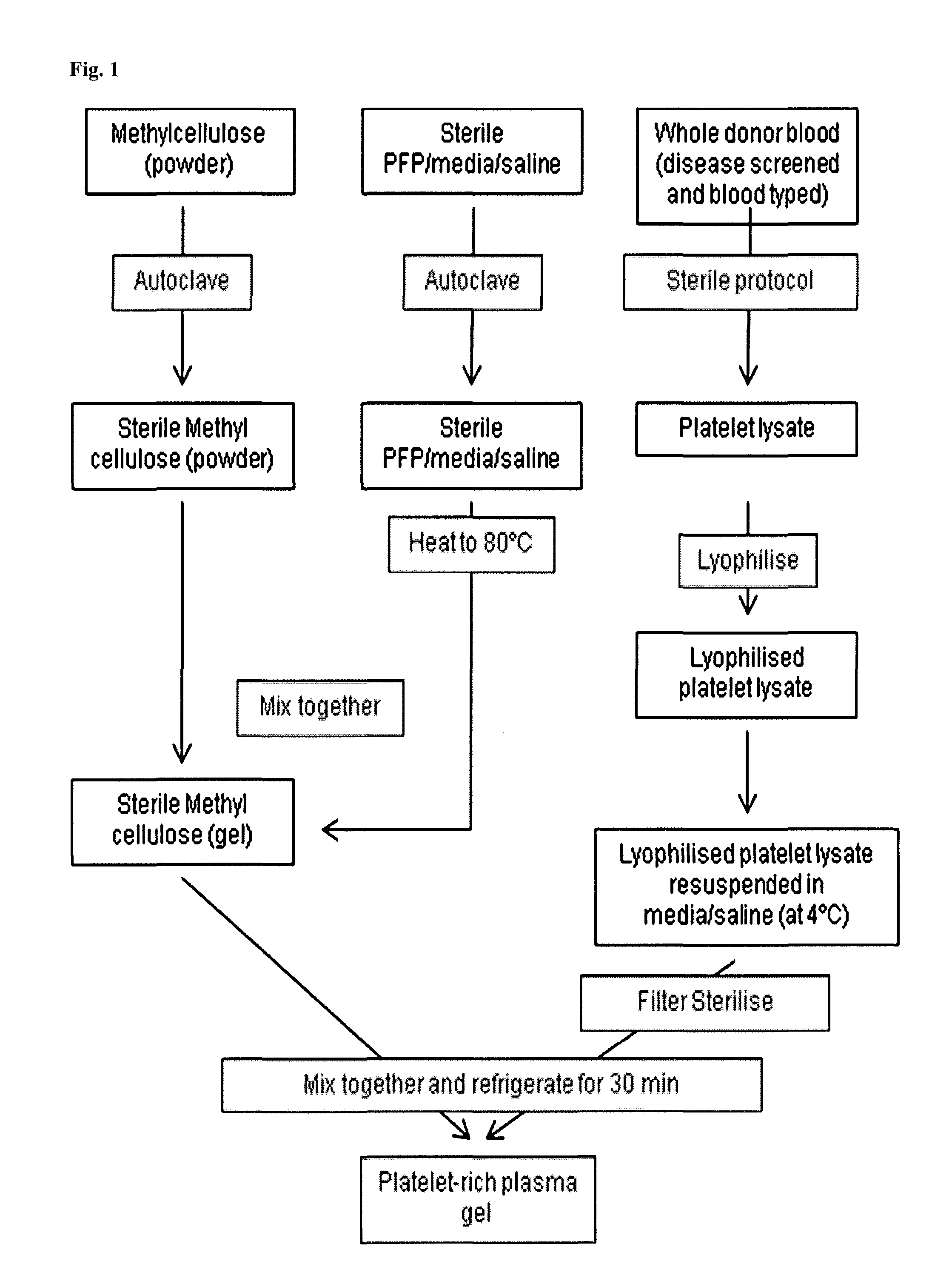
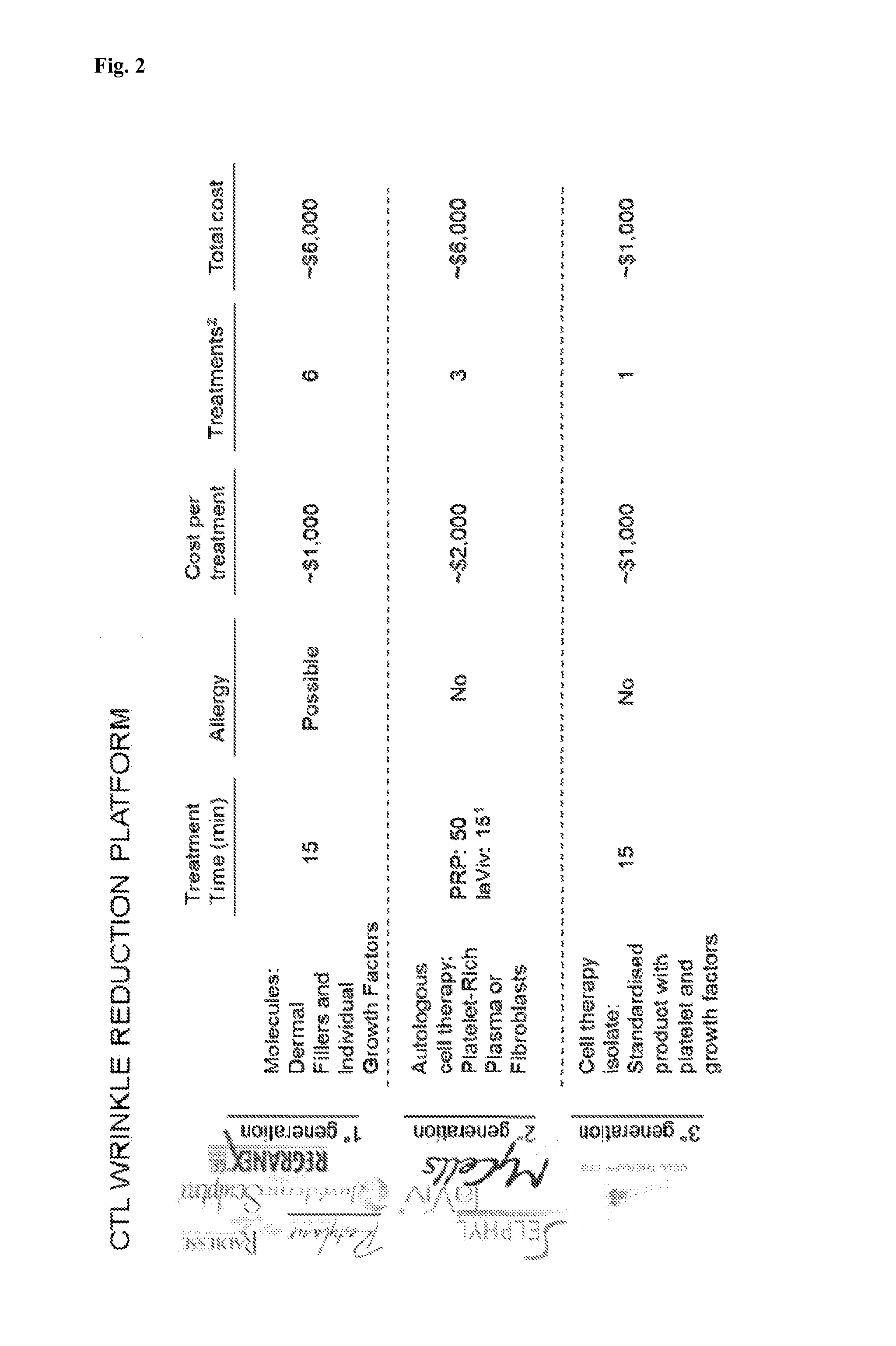
Patents
Literature
119 results about "Platelet lysate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
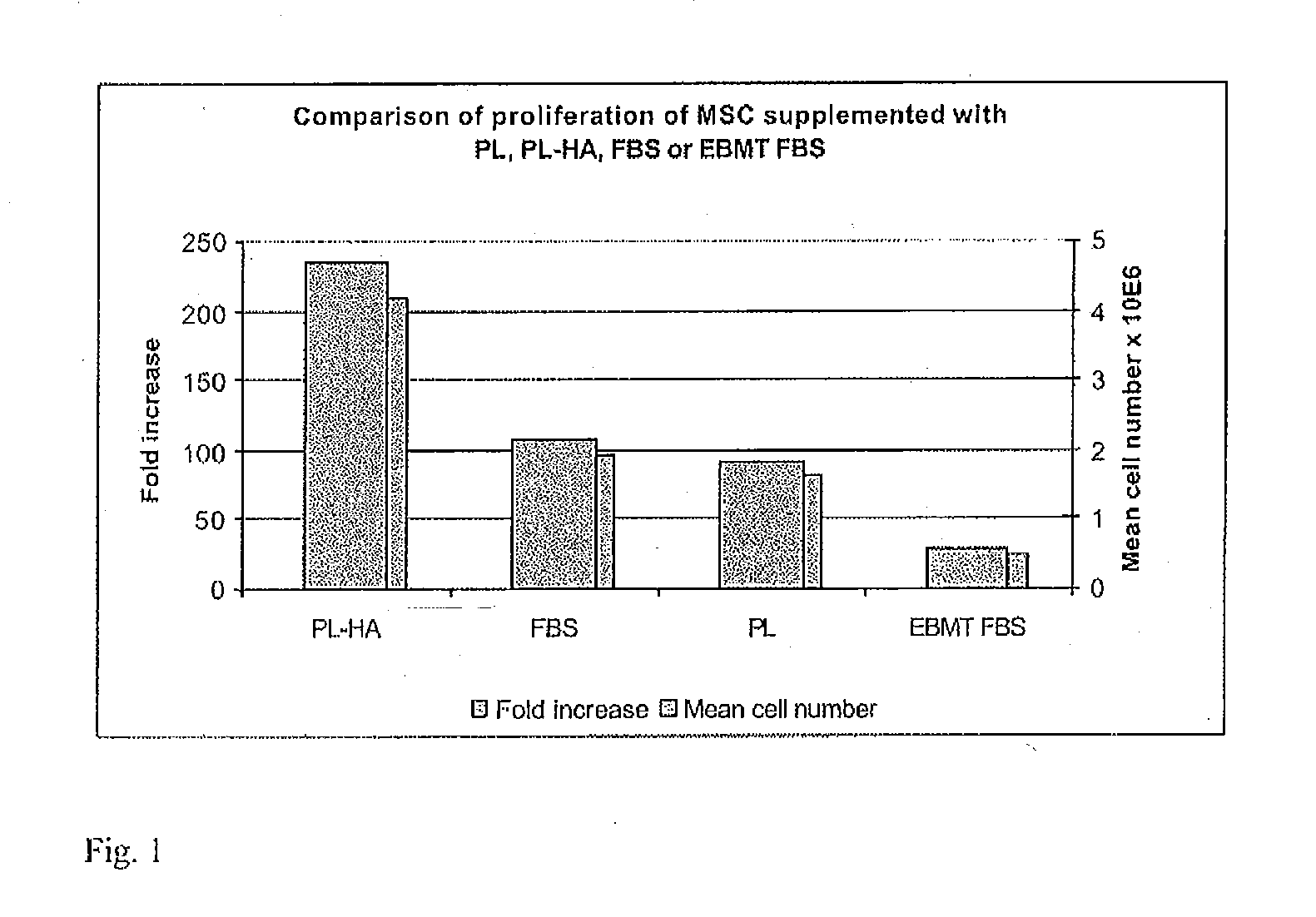
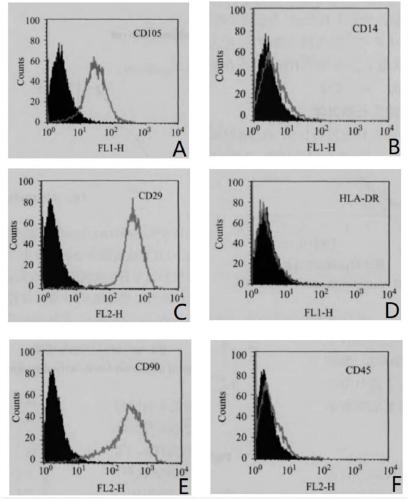
Human platelet lysate (or hPL) is a substitute supplement for fetal bovine serum (FBS) in experimental and clinical cell culture. It is a turbid, light-yellow liquid that is obtained from human blood platelets after freeze/thaw cycle(s). The freeze/thaw cycle causes the platelets to lyse, releasing a large quantity of growth factors necessary for cell expansion. FBS-free cell culture media, e.g. with platelet lysate or chemically defined/ animal component free, are used for cell therapy or regenerative medicine. They are commercially available in GMP (good manufacturing practice)-quality which is generally basis for regulatory approval.
Method for cultivating autologous umbilical cord mesenchymal stem cells by adopting human umbilical cord blood rich platelet lysate
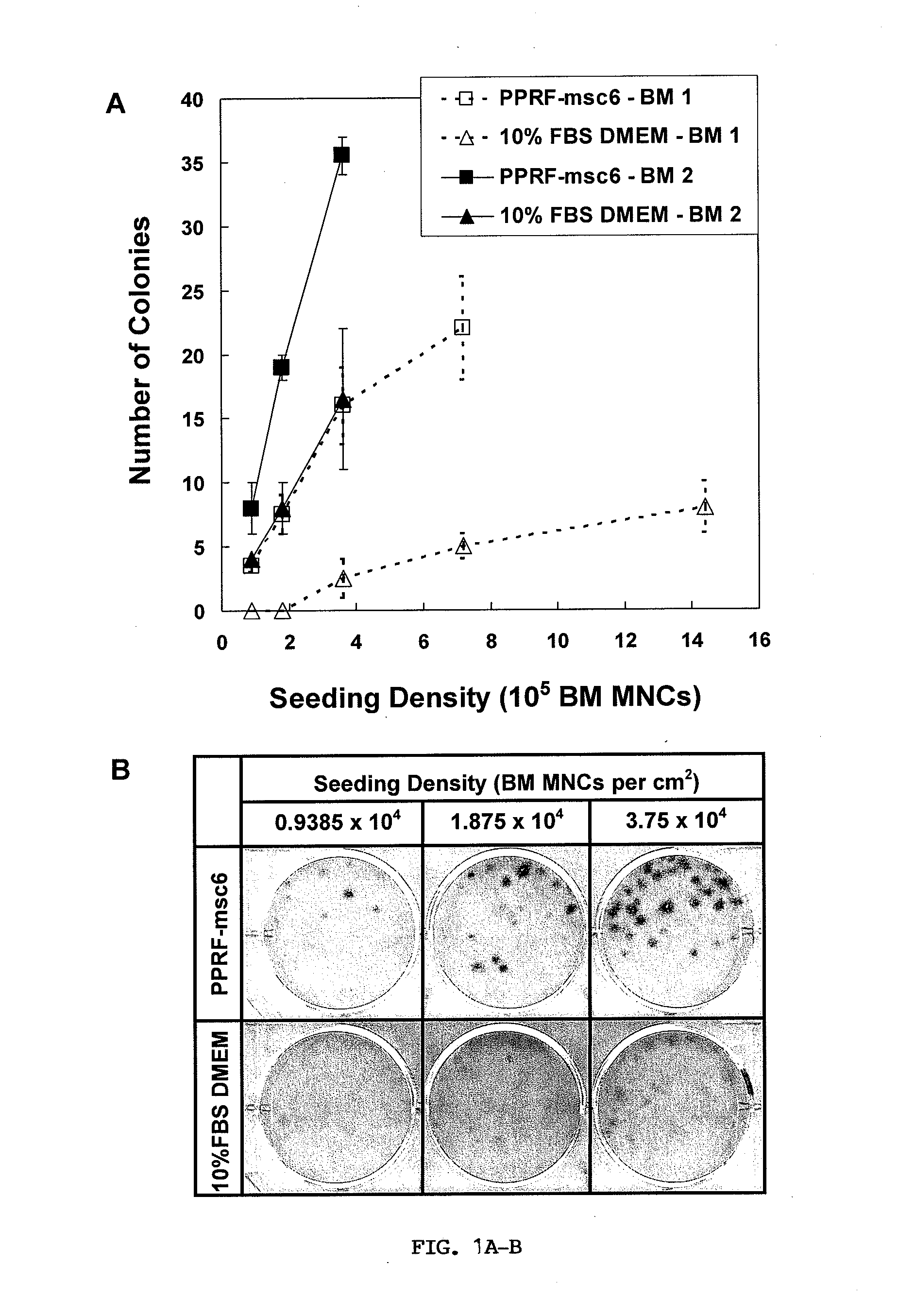
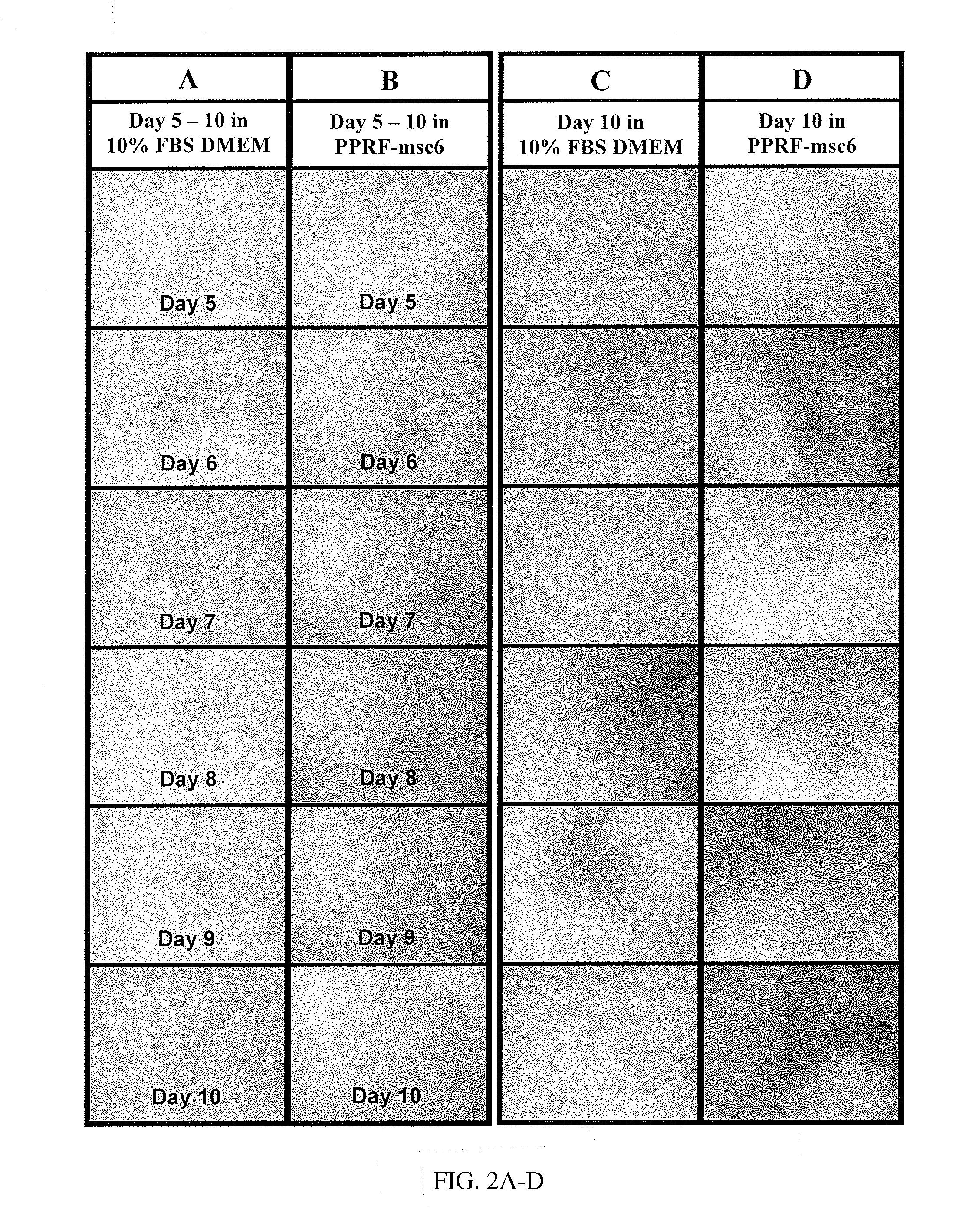
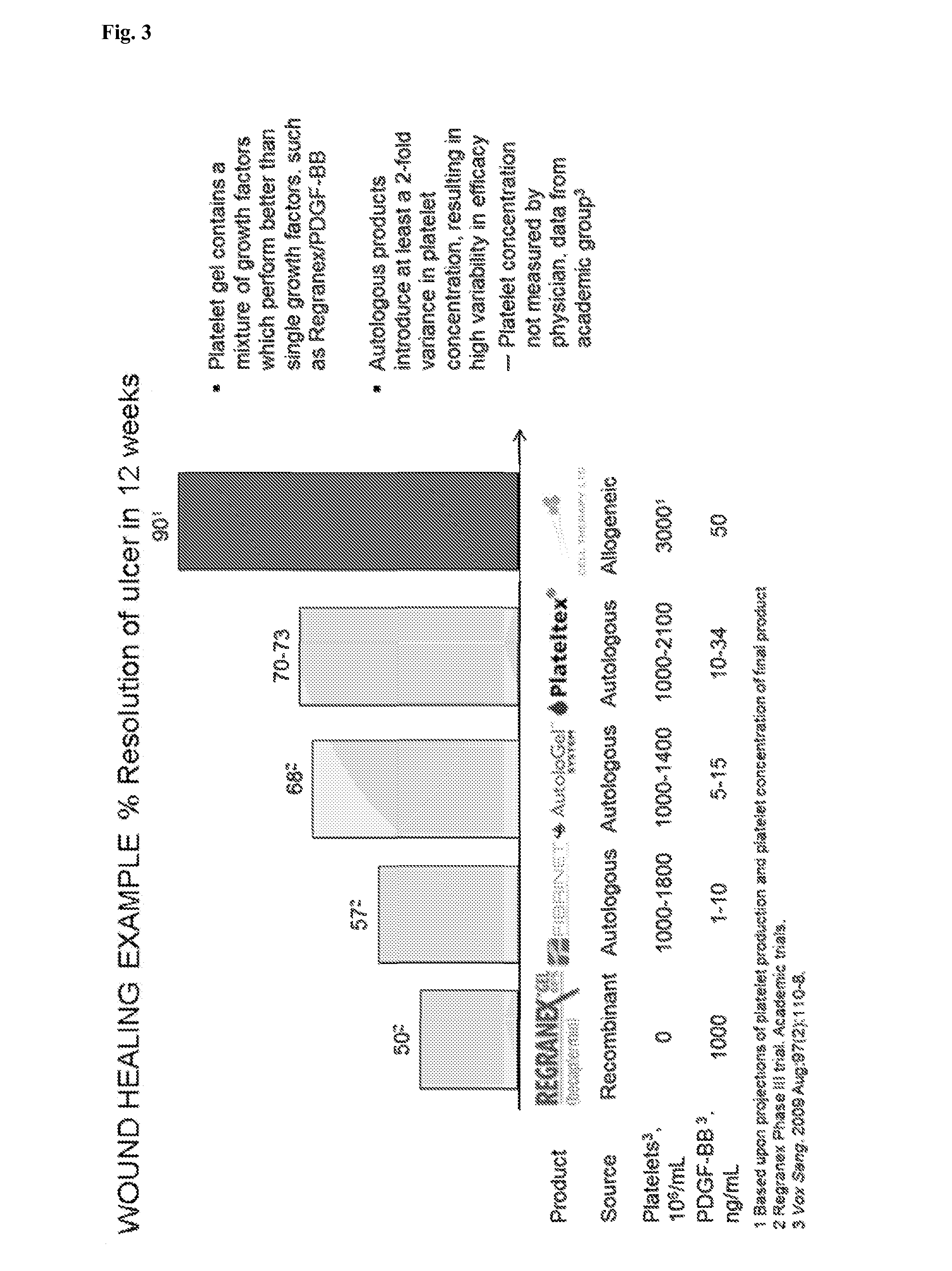
ActiveCN103352026ADoes not affect storageNormal colonySkeletal/connective tissue cellsBlood/immune system cellsCryopreservationMonosomal karyotype
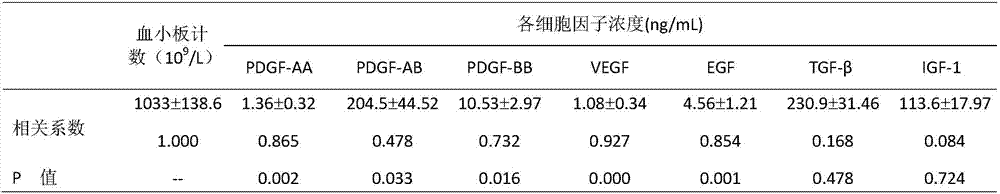
The invention relates to a method for cultivating autologous umbilical cord mesenchymal stem cells by adopting human umbilical cord blood rich platelet lysate. The conventional cell bank preparing processes adopt blood serum of calves or fetal calves to cultivate, digest, cryopreserve and the like, so that the risk of heterogeneous protein residue certainly exists in the application of clinic umbilical cord stem cells in the future. The method comprises the following steps of umbilical cord blood rich platelet serum preparation, platelet lysate preparation, preparation of serous without platelet, the confirmation of the content of cell factors, namely PDGF-AB, FGF2, TGF-Beta and VFGF in the platelet lysate, the separation and the primary culture of umbilical cord stem cells, the culture and passage of umbilical cord stem cells, the cryopreservation of umbilical cord stem cells, and the karyotype analysis. The method is used for cultivating stem cells.
Owner:武汉天晴干细胞有限公司
Methods and Compositions for Isolating, Maintaining and Serially Expanding Human Mesenchymal Stem Cells
InactiveUS20100015710A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSodium bicarbonateLipid formation
Compositions and methods for isolating and expanding human mesenchymal stem / progenitor cells through multiple passages in defined serum-free environments are provided. The culture media compositions includes a basal medium supplemented with a nutrient mixture such as Ham's F12 nutrient mixture, glutamine, buffer solutions such as sodium bicarbonate and hepes, serum albumin, a lipid mixture, insulin, transferrin, putrescine, progesterone, fetuin, hydrocortisone, ascorbic acid or its analogues such as ascorbic acid-2-phosphate, fibroblast growth factor and transforming growth factor β, and are free of serum or other undefined serum substitutes such as platelet lysate. Methods employing these compositions and protein-coated surfaces for the isolation of mesenchymal stem / progenitor cells from human bone marrow and other tissues such as adipose tissue are also provided. Finally, methods are also provided for serially expanding these cells through multiple passages without losing mesenchymal stem cell-specific proliferative, phenotypical and differentiation characteristics.
Owner:UTI LLP
Mesenchymal stem cell self-preserving liquid
ActiveCN102349500AAdapt to the living environmentMaintain acid-base balanceDead animal preservationVitamin injectionPeroxidase
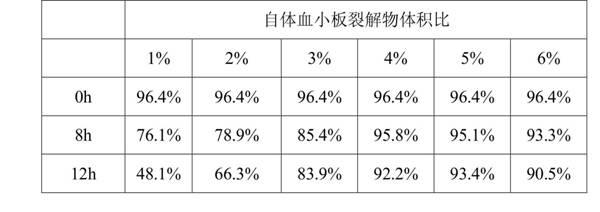
The invention discloses a mesenchymal stem cell self-preserving liquid which is prepared from the following raw materials according to volume ratios: 1-6% of self platelet lysate, 95-97% of solution medium and 0-1% of vitamin C injection, wherein the solution medium is a mixed sugar electrolyte injection or physiological saline injection. The mesenchymal stem cell self-preserving liquid has the advantages that: growth factors contained in the mesenchymal stem cell self-preserving liquid can maintain the activity state of cells, the vitamin C can maintain the activity of various peroxidases, at the same time, the mixed sugar electrolyte injection is especially suitable for cell preservation, and osmotic pressure is also favorable for nutrient absorption of the cells so as to carry out metabolism; the activity of the mesenchymal stem cells which are preserved in the mesenchymal stem cell self-preserving liquid within 8 hours is still larger than 90%, the cell activity change is not larger than 7%, and the activity of the mesenchymal stem cells which are preserved in the mesenchymal stem cell self-preserving liquid within 12 hours is still maintained at 85%; and by using the mesenchymal stem cell self-preserving liquid, the requirements for human intravenous back-transfusion safety can be met, and the cell preserving problem of long-time transport is solved, thus more patients have opportunity to undergo mesenchymal stem cell treatment.
Owner:CHENGDU QINGKE BIOTECH
Method for preparing platelet lysate and application of platelet lysate
InactiveCN104673747APromote activationPromote growthArtificial cell constructsSkeletal/connective tissue cellsFreeze thawingCell growth
The invention relates to the field of cytobiology and in particular relates to a method for preparing platelet lysate and application of the platelet lysate. The preparation method comprises the following steps: oscillating platelets at the temperature of 20-24 DEG C, preserving heat for 1-5 days, treating by adopting a repeated freeze-thaw method and an ultrasonic method, centrifuging, collecting the supernatant, and removing the fibrous proteins, thereby obtaining the platelet lysate. According to the method for preparing the platelet lysate provided by the invention, the content of cell factors in the platelet lysate can be obviously improved, quantitative balance of the cell factors can be realized, the differences among the product batches are reduced, and the effect of stably promoting human-derived cell growth can be achieved.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Cell frozen preservation solution and cell frozen preservation method
ActiveCN107027743AVulnerableDoes not affect differentiation abilityDead animal preservationMedicineCell culture media
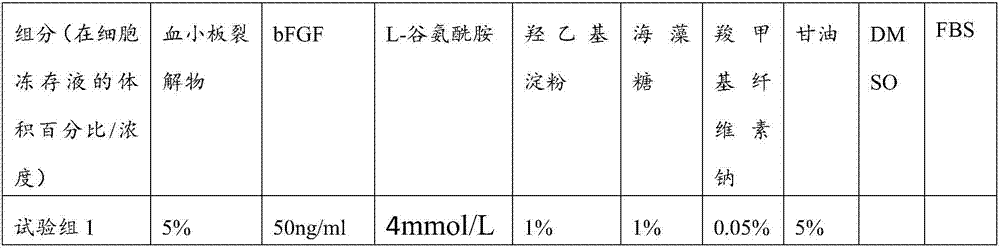
The invention belongs to the field of cell frozen preservation, particularly relates to a cell frozen preservation solution and a cell frozen preservation method and provides a cell frozen preservation solution. The cell frozen preservation solution comprises a basic culture medium, a blood platelet lysate, bFGF and L-glutamine. The cell frozen preservation solution does not contain animal serum or DMSO, the possibility that an exogenous virus can be introduced by the animal serum is eliminated and the bad effect of the DMSO on adipose-derived stem cells is eliminated. Furthermore, the invention further provides the cell frozen preservation method. According to the method, complicated programmed cooling is not needed and operation is simple. After the adipose-derived stem cells are frozen and preserved by adopting the cell culture medium and the cell frozen preservation method for a year, the survival rate of the cells can reach 93% and the differentiation capacity of the adipose-derived stem cells is not affected.
Owner:沃昕生物科技(深圳)有限公司
Plasma-free platelet lysate for use as a supplement in cell cultures and for the preparation of cell therapeutics
InactiveUS20120276632A1Effective trainingCulture processCell culture mediaCell culture mediaBlood plasma
The present invention provides a cell culture medium supplement comprising plasma-free platelet lysate and medium supplemented with this supplement. The present invention further provides a method for preparing the supplement comprising the steps of (a) preparing platelet rich plasma; (b) removing the plasma; and (c) lysing the platelets.
Owner:MEDICAL UNIV OF GRAZ
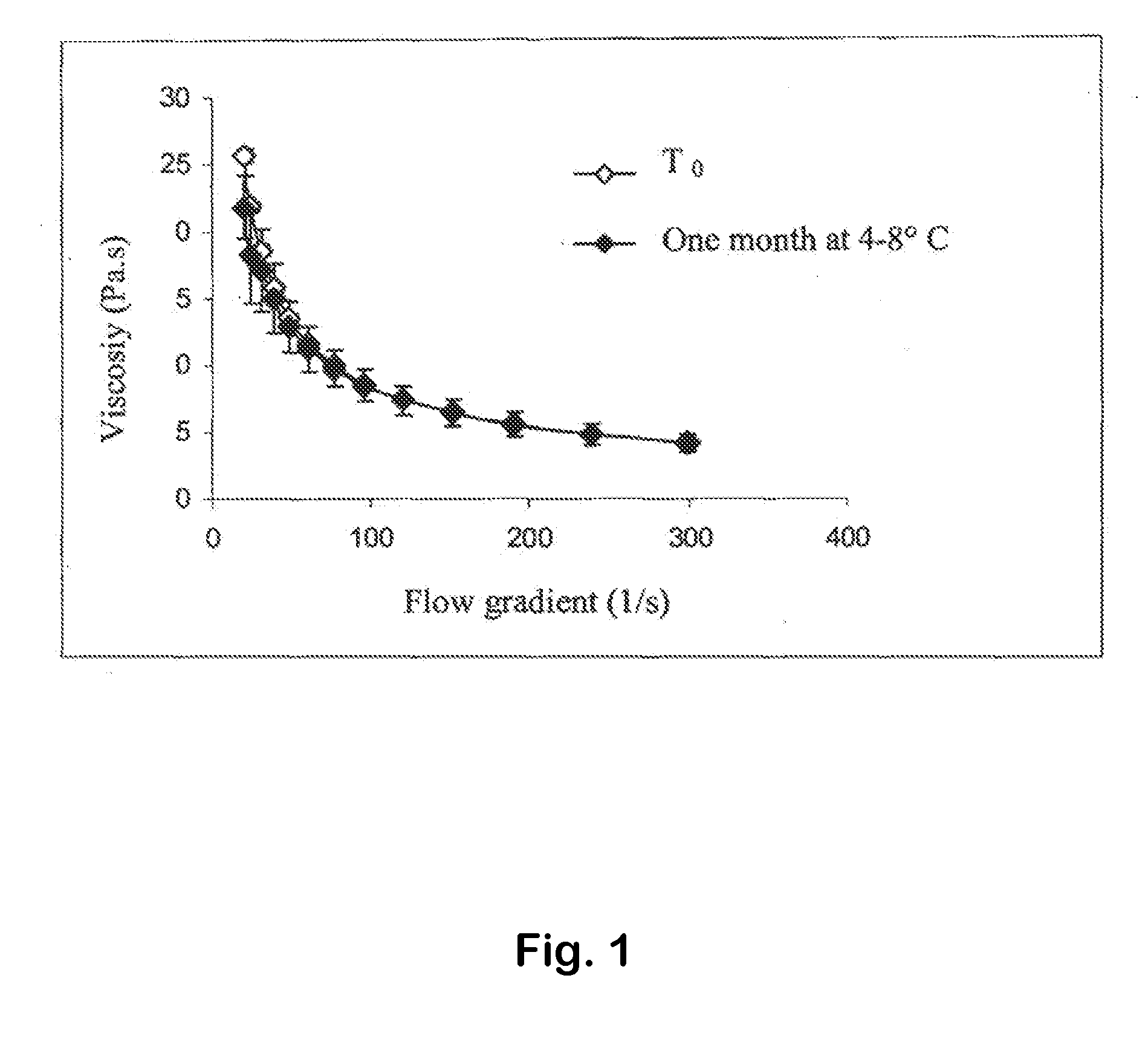
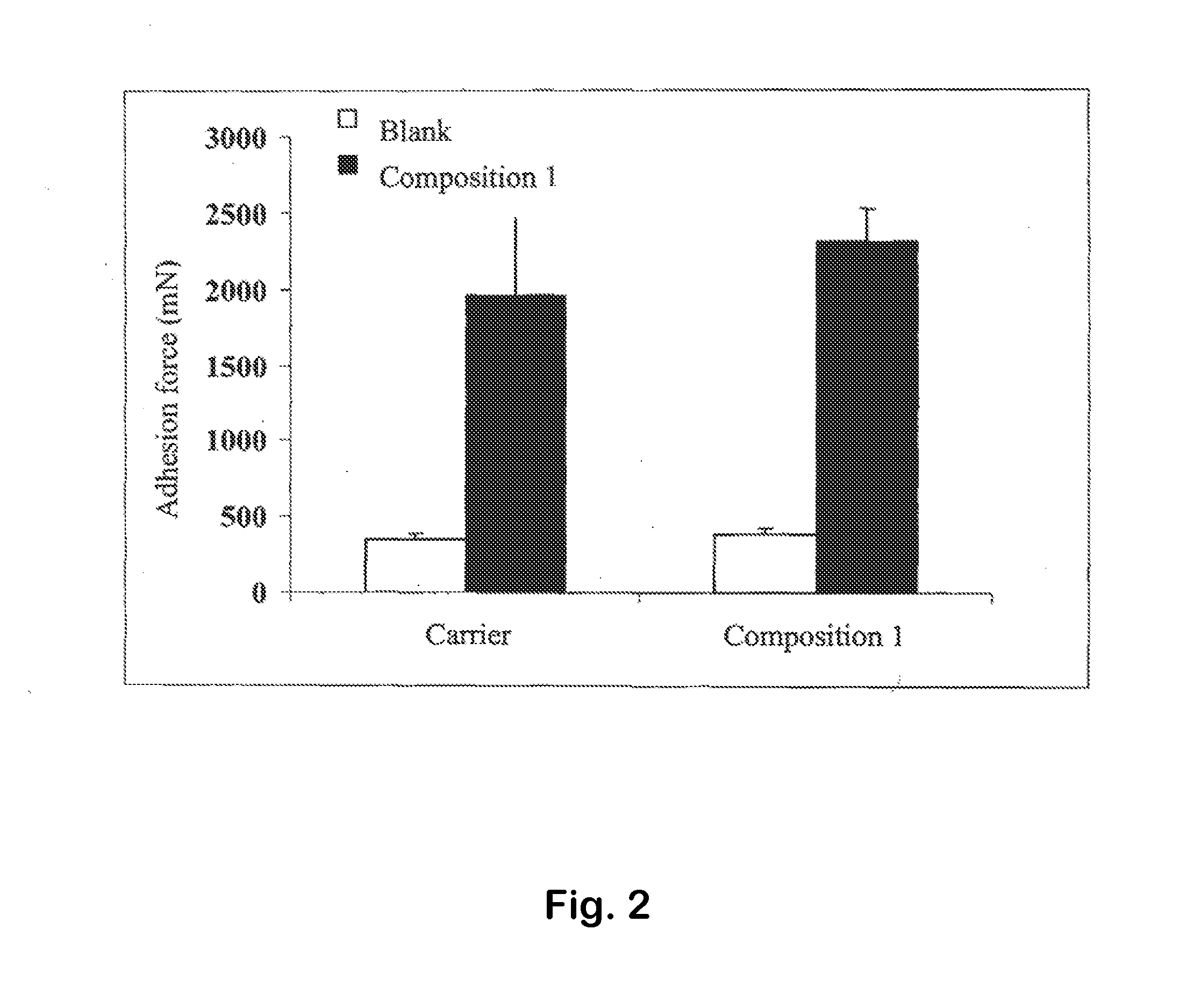
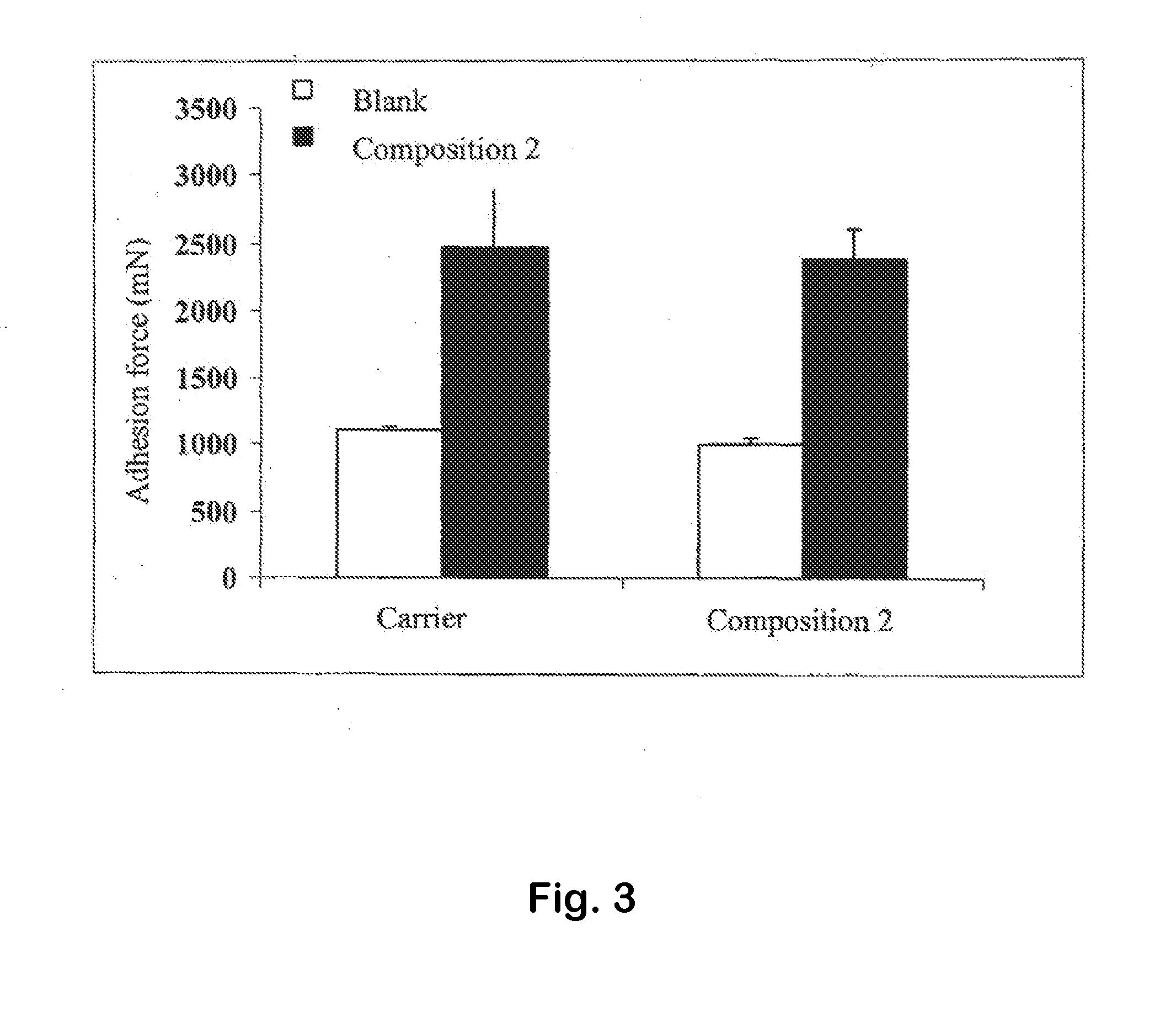
Platelet Lysate and Bioadhesive Compositions Thereof for the Treatment of Mucositis
Owner:BIOMED DEVICE
Plasma-free platelet lysate for use as a supplement in cell cultures and for the preparation of cell therapeutics
InactiveUS20090305401A1Effective trainingCulture processCell culture mediaCell culture mediaBlood plasma
The present invention provides a cell culture medium supplement comprising plasma-free platelet lysate and medium supplemented with this supplement. The present invention further provides a method for preparing the supplement comprising the steps of (a) preparing platelet rich plasma; (b) removing the plasma; and (c) lysing the platelets.
Owner:STRUNK DIRK +2
Compositions containing platelet contents
This document provides methods and materials relating to platelet lysates. For example, methods and materials for using platelet lysate compositions to grow adult stem cells, to differentiate adult stem cells, to grow primary cell cultures, to grow tumor stem cells, and to identify effective growth factors are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Flow micro-sphere method for detecting plastocyte specificity immune body
InactiveCN101246173AIncreased sensitivityContribute to basic experimental researchMaterial analysisHuman plateletMicrosphere
The invention provides a method belonging to the field of cytometric bead array with high sensitivity for testing platelet specific antibody based on platelet basic experimental study. Platelet lysate is used to incubate the microsphere which is coated with platelet membrane glycoprotein monoclonal antibody, then goat-anti-human IgG polyclonal antibody labeled with phycoerythrin is added, which is analyzed in cytometric bead array. If autoantibodies exist on the surface of platelet, 'microsphere-platelet membrane glycoprotein monoclonal antibody-platelet membrane glycoprotein autoantibodies-goat-anti-human IgG polyclonal antibody labeled with phycoerythrin' complex structure is formed, the fluorescence intensity of the test microsphere is enhanced. The invention is easy to operate, and has mature technology, high sensitivity for testing platelet autoantibodies, which is good for basic experimental study of platelet antibody.
Owner:侯明 +2
Mesenchymal stromal cell populations and methods of using same
InactiveUS20110123498A1Reduce the possibilityMinimize damageBiocideMammal material medical ingredientsStromal cellKidney
Owner:ALLOCURE
Mesenchymal stem cell serum-free medium and cell isolation and cultivation methods
PendingCN105950550ASimple ingredientsEliminate distractionsCulture processSkeletal/connective tissue cellsCells isolationSerum free media
The invention provides a mesenchymal stem cell serum-free medium. The mesenchymal stem cell serum-free medium is prepared from, by volume, 94-97 parts of DMEM / F12, 2-5 parts of human blood platelet lysate and 1 part of non-essential amino acid. The invention further provides application of the medium and isolation and cultivation methods of the mesenchymal stem cell serum-free medium. The serum-free medium can be used for effectively separating and cultivating mesenchymal stem cells derived from placentae and umbilical cords, the effects are superior to commercially available media, the cost is low, the safety is high, and the application prospect is good.
Owner:SICHUAN NEO LIFE STEM CELL BIOTECH
Tissue and/or cell cryopreservation protective solution as well as preparation and application thereof
ActiveCN108617638AShow Immunofluorescence ResultsCosmetic preparationsToilet preparationsBiologyPlatelet lysate
The application provides a tissue and / or cell cryopreservation protective solution as well as preparation and application thereof. The tissue and / or cell cryopreservation protective solution comprisesa permeating protective agent and a non-permeating protective agent, wherein the cryopreservation protective solution further comprises platelet lysates. In addition, the application further providesa method for cryopreserving tissues and / or cells by using the tissue and / or cell cryopreservation protective solution provided by the application, as well as a cryopreserved tissue and / or cell and use of the cryopreserved tissue and / or cell.
Owner:BIOCELLS BEIJING BIOTECH CO LTD
Blood platelet lysate and method of producing the same
A method of preparing a blood platelet lysate starting from platelet-rich plasma from whole blood of animals, to which blood a citrate has been added to prevent coagulation, is described. The method is characterised by concentrating the platelet-rich plasma by ultrafiltration to obtain a plasma that is richer in platelets, adding water for lysis of the platelets included, adding Ca2+ to the lysed concentrated platelet-rich plasma for forming coagel of other components than lysed platelets, centrifuging the coagel, whereby a clear, bright-red liquid of blood platelet lysate is obtained, which substantially consists of lysed platelets, and a coagulate, and, after centrifugation, the liquid going through a filtering step.
Owner:PROLIFF
Preparation method of serum-free freezing medium of human umbilical cord mesenchymal stem cells (hUC-MSCs)
The invention relates to the technical field of freezing mediums, and discloses a preparation method of a serum-free freezing medium of human umbilical cord mesenchymal stem cells (hUC-MSCs). The serum-free freezing medium of the hUC-MSCs is prepared from the following components in percentage by volume: 20-80% of a basal culture medium, 5-30% of platelet lysate, 1-10% of instant-dissolving protein polysaccharide mother liquor, and 5-25% of trehalose mother liquor. According to the preparation method of the serum-free freezing medium of the hUC-MSCs, the platelet lysate and trehalose are usedfor replacing original animal serum and DMSO, and thus the preparation method has the advantages that the activity of the UC-MSCs can be maintained for a long time, the survival rate of the cells is significantly increased, and preparation is easy and safe; and the problems that the DMSO serves as a permeating protective agent and can penetrate into the cells quickly, the time that the cells are exposed in the harmful environment is shortened, however, the DMSO has the toxic effect on the cells at the room temperature, operation at the room temperature needs to be quick as soon as possible, FBS can increase the probability of animal pathogenic pollution, and consequently, uncertain factors are added for future using of the cells are solved.
Owner:青岛麦迪赛斯生物科技有限公司
Method for preparing PL (platelet lysate)
PendingCN107384856AFully lysedReduce wasteCulture processSkeletal/connective tissue cellsPL ExtractCulture cell
The invention discloses a method for preparing a PL (platelet lysate) and an application of the PL. The method for preparing the PL comprises the following steps: concentrated platelets are centrifuged under a centrifuge at 800-1000 rpm for 15-25 min, the upper layer is platelet-rich plasma and the bottom layer is a red blood cell layer after centrifugation, and the red blood cell layer at the bottom is separated out; the upper platelet-rich plasma is mixed thoroughly in batches, and platelets are sufficiently lysed with a multigelation process; cell debris is removed, and the final PL is obtained. The invention also provides a cell culture method for culturing cells with the prepared PL. With adoption of the method, the yield of PL extracted from the concentrated platelets can be increased to 95%, operation time is shortened, and clinical expired products can be repeatedly used and developed.
Owner:重庆赛纳思生物科技有限公司
Mesenchymal stromal cell populations and methods of isolating and using same
InactiveUS20110293576A1Minimize damageIncrease pressureBiocideNervous disorderCulture cellStromal cell
Owner:ALLOCURE
Efficient mesenchymal stem cell culture solution without serum component
InactiveCN109402050APromote growthGuaranteed feasibilitySkeletal/connective tissue cellsCell culture active agentsIfn alphaHuman platelet
The invention relates to the technical field of biology, in particular to a mesenchymal stem cell culture solution. The mesenchymal stem cell culture solution is prepared from the following components: human platelet lysate, mycillin (Pen Strep), long-acting glutamine, a chemotactic factor XCL1, a chemotactic factor CCL3, a heat shock protein (HSP70), a telomerase inhibitor IFN-alpha 2b and a basal culture medium DMEM. The components of the stem cell culture solution are wide in source, cultured mesenchymal stem cells are high in purity, quick in proliferation and good in dryness, and the mesenchymal stem cell culture solution is suitable for extracorporeal large-scale culture to conduct preclinical study and related clinical study.
Owner:沈阳中心血站
Platelet lysate gel
InactiveUS20140335195A1Easy to adaptCosmetic preparationsToilet preparationsWrinkle skinVaginal atrophy
The invention concerns a pharmaceutical composition comprising a platelet lysate and its use to treat a wound, an anal fissure, vaginal atrophy or a wrinkle.
Owner:CELL THERAPY LTD
Stem cell culture medium as well as preparation method and application thereof
InactiveCN108220231APromote growthGuaranteed purityCulture processSkeletal/connective tissue cellsSerum free mediaStem cell culture
The invention belongs to the technical field of cell culture, and in particular relates to a stem cell culture medium as well as a preparation method and application thereof. The stem cell culture medium provided by the invention comprises a mesenchymal stem cell serum-free medium, platelet lysate, glutamine, non-essential amino acids and a hepatocyte growth factor, wherein the mesenchymal stem cell serum-free medium and platelet lysate enable menstrual blood stem cells to rapidly grow, the glutamine and the non-essential amino acids provide the nutrients needed by the menstrual blood stem cells, and the hepatocyte growth factor stimulates the proliferation of the menstrual blood stem cells. The stem cell culture medium solves the technical problem that the stem cell culture medium in theprior art is not suitable for culturing the menstrual blood stem cells.
Owner:GUANGZHOU ZISHENG BIOLOGICAL TECH CO LTD
Culture medium and cultural method for endometrial stem cells
ActiveCN109593706APlay a role in purificationPromote wall growthCulture processArtificial cell constructsSerum free mediaBiotechnology
The invention belongs to the field of biotechnology, in particular to a culture medium and a cultural method for endometrial stem cells. The culture medium provided by the invention comprises a serum-free medium, a blood platelet lysate, N-acetylcysteine, galacturonic acid and an antibiotic. The culture medium and the cultural method for endometrial stem cells provided by the invention are used for solving the technical defects of low value increase efficiency of the endometrial stem cells, low cell quality and easy differentiation caused by existing culture medium culture.
Owner:GUANGDONG CHINAHEALTH LIFE SCI CO LTD
Culture system beneficial for mesenchymal stem cells to be applied in bone regeneration
InactiveCN106701671ACulture processSkeletal/connective tissue cellsBone tissue engineeringBone defect
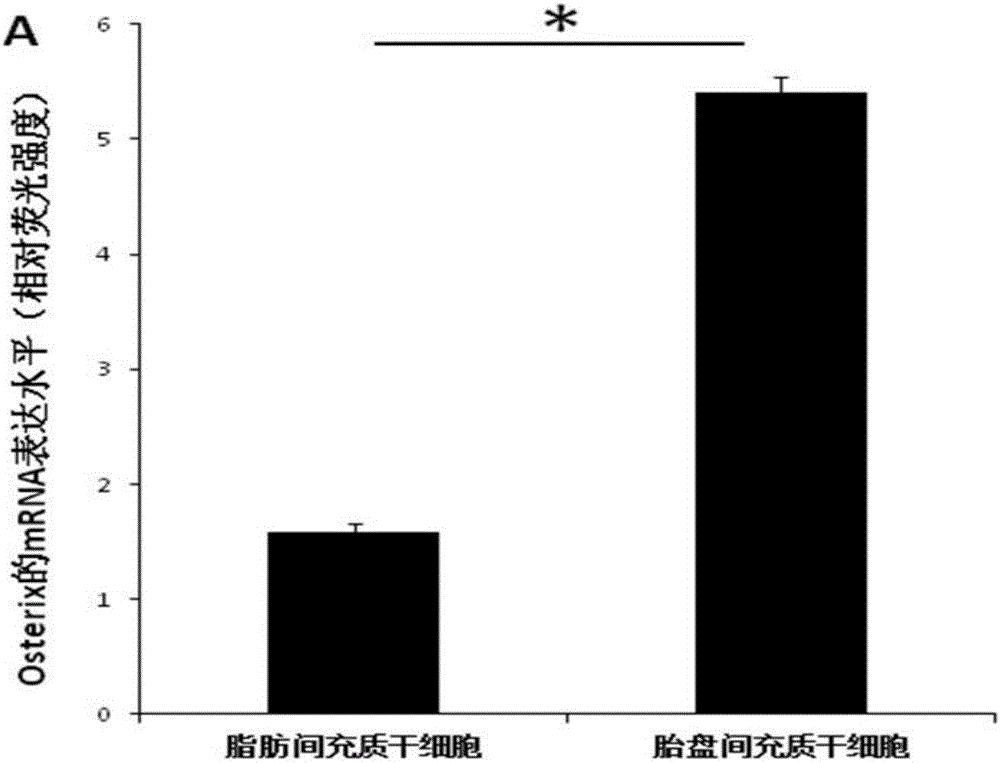
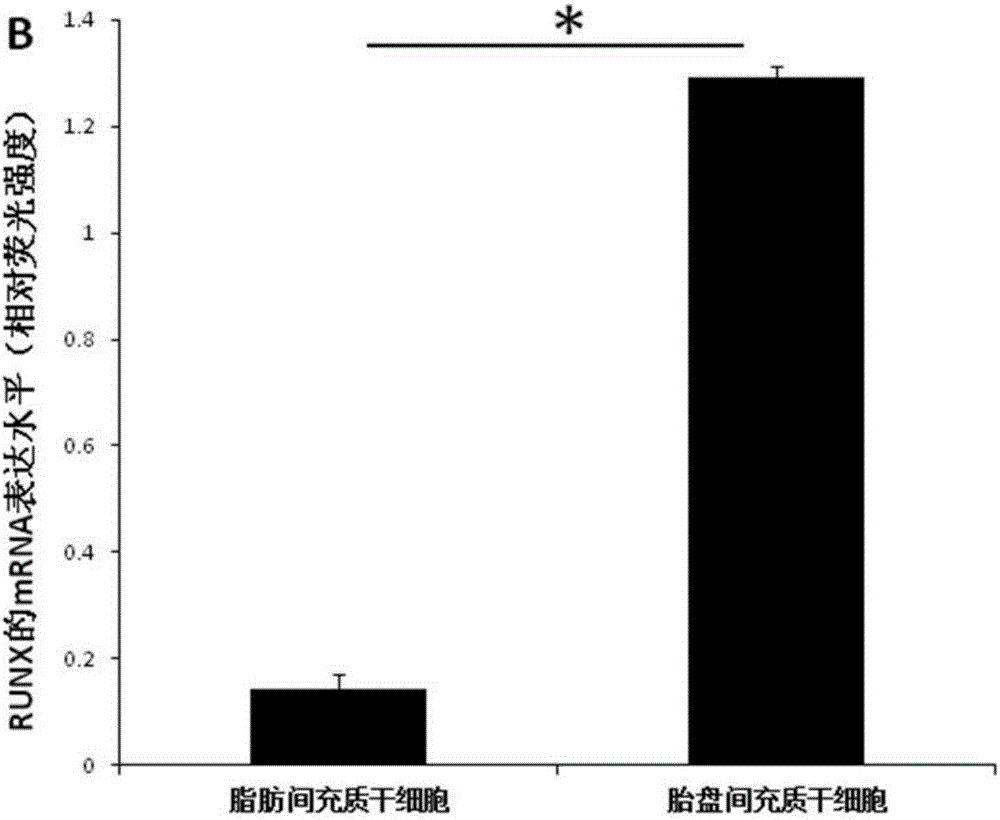
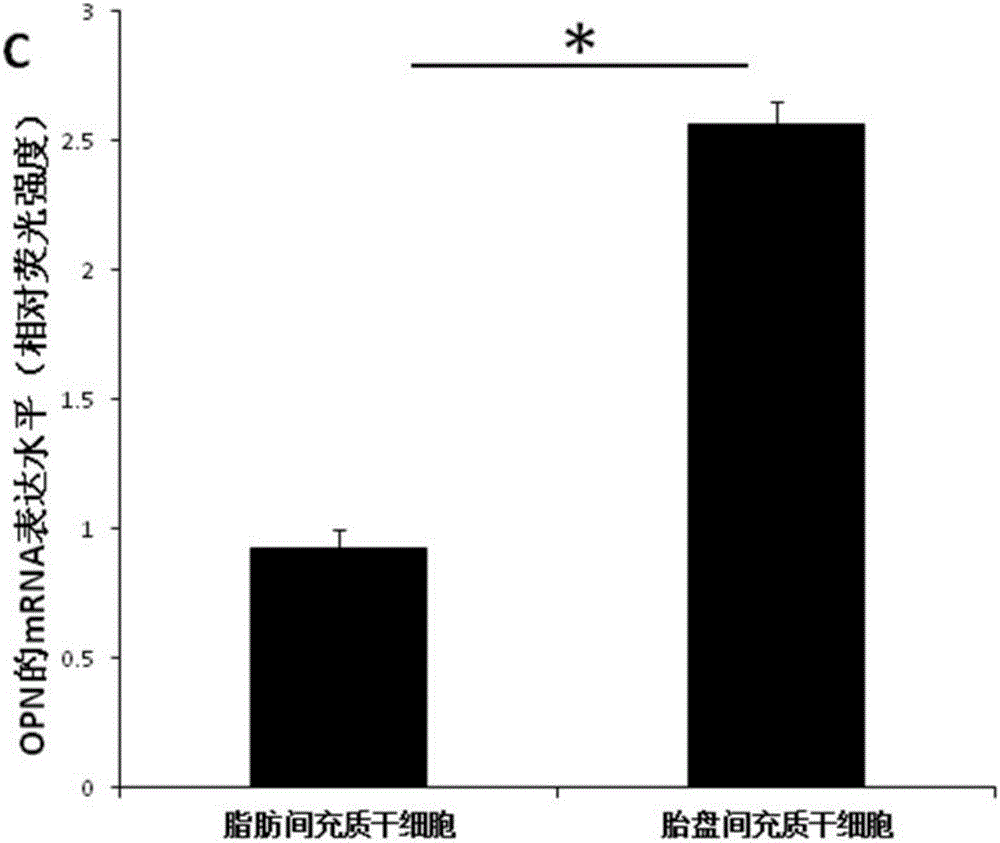
The invention relates to a culture system beneficial for mesenchymal stem cells to be applied in bone regeneration, wherein the culture system includes a-MEM+5% platelet lysate+50 mg / ml ascorbic acid +10 nmol / L EGF+10 nmol / L bFGF+10 nmol / L recombinant human brain natriuretic peptide. Placenta-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells cultured by the culture system all can be used as seed cells to be applied in bone tissue engineering treatment of bone defects, the step of mesenchymal stem cell in-vitro osteogenic induction is avoided, and the time for clinical application of bone tissue engineering treatment of bone defects is shortened by at least 14 days. In addition, compared with the adipose-derived mesenchymal stem cells, with application of the cultured placenta-derived mesenchymal stem cells, a bone regeneration factor gene is significantly highly expressed.
Owner:TIANJIN KANGTING BIOLOGICAL ENG GRP CO LTD
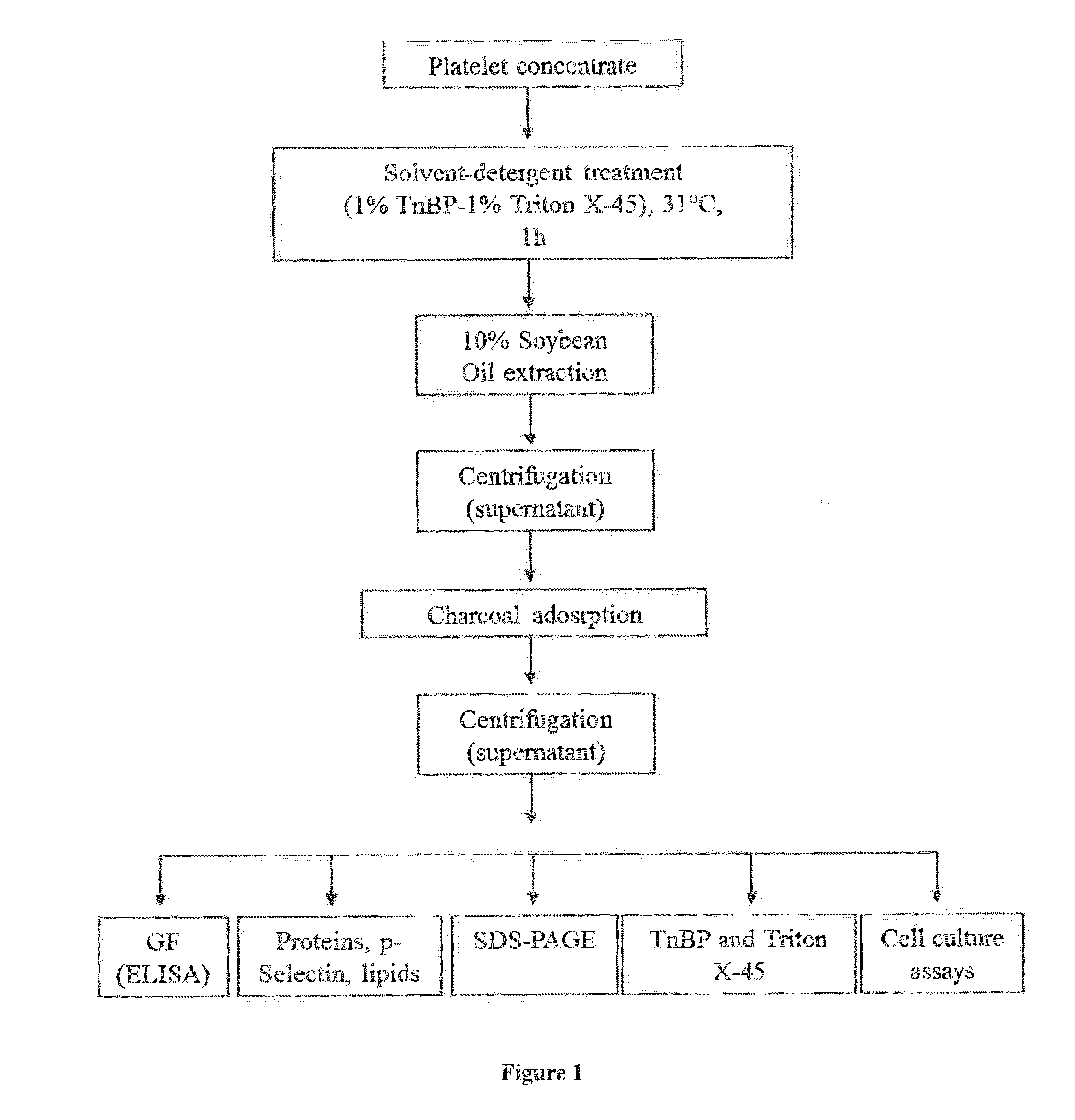
Virally-Inactivated Growth Factors-Containing Platelet Lysate Depleted of PDGF and VEGF and Preparation Method Thereof
The invention concerns human platelet extracts rich in growth factors (PGF) for wound healing and stem cell expansion. Accordingly the subject invention relates to a virally-inactivated growth factors-containing platelet lysate depleted of PDGF and VEGF, which is preferably enriched in TGF, IGF and EGF-rich. The present invention further concerns a method for obtaining a platelet lysate comprising the steps of contacting a starting platelet concentrate with a solvent and / or a detergent, incubating the starting platelet concentrate with the solvent and / or detergent for a period of at least 5 minutes to 6 hours, at a pH maintained in a range from about 6.0 to about 9.0, and at a temperature within the range of from 2° C. to 50° C., optionally removing the solvent and / or the detergent by oil extraction and obtaining an aqueous protein phase, and incubating the solvent and / or detergent-treated platelet concentrate or the aqueous protein phase with charcoal.
Owner:ZHENG YANG BIOMEDICAL TECH
Stem cell culture medium and stem cell separating method
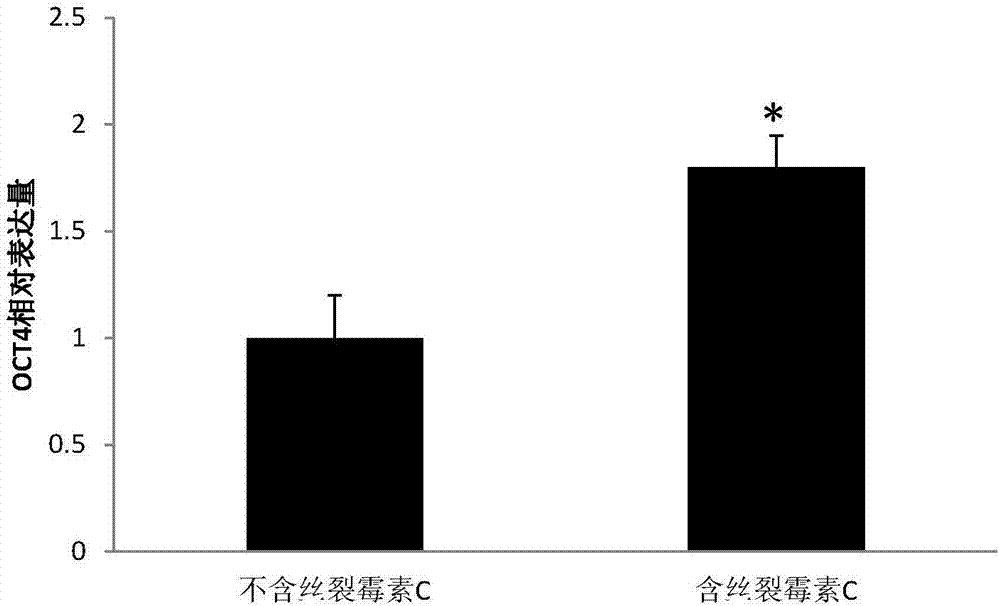
ActiveCN107083359AEliminate bad effectsAvoid introducingCell dissociation methodsCulture processMitomycin CCell culture media
The invention belongs to the field of stem cell culture, and particularly relates to a cell culture medium and a stem cell culture method. The invention provides a stem cell culture medium. The stem cell culture medium comprises a basic culture medium, a platelet lysate, an L-Glutamine and a mitomycin c. The invention also provides a stem cell separating method. The stem cell separating method comprises the following steps that the in-vitro tissue and the stem cell culture medium are mixed to be placed in a culture flask for culturing until cells creep out from the in-vitro tissue, the in-vitro tissue is scraped; the culture of the cells is continued, when the fusion degree of stem cells in the culture flask reaches 80% to 90%, the cells are passage-cultured after digestion. The stem cell culture medium can avoid the risk that the existing stem cell culture medium contains mostly animal serum so as to introduce exogenous virus; the stem cell separating method is simple to operate.
Owner:沃昕生物科技(深圳)有限公司
Method of in-vitro amplification and purification culture of mesenchymal stem cells
InactiveCN104480068AReduce harmLow costSkeletal/connective tissue cellsFluid replacementCell culture media
The invention relates to a method of in-vitro amplification and purification culture of mesenchymal stem cells in differentiation from mesenchymal stem cells to osteoblasts. A used cell culture medium comprises the following components: 5-20% of PL platelet lysate, 2-5U / ml of heparin, 5-10microgram / ml of doxycycline, and a basic culture medium DMEM or a basic culture medium AMEM. According to the method disclosed by the invention, the method comprising steps of separation and extraction of mesenchymal stem cells, cell inoculation, cell culture, cell culture fluid replacement and cell propagation are adopted, and the obtained mesenchymal stem cells are high in purity, high in in-vitro amplification capacity, and good in the capacity of differentiation to osteoblasts.
Owner:黄相杰
Cell culture solution for enhancing cartilage differentiation induction, method and application
PendingCN111826343APromote growthPromote proliferationCulture processSkeletal/connective tissue cellsChondroblast differentiationPlatelet lysate
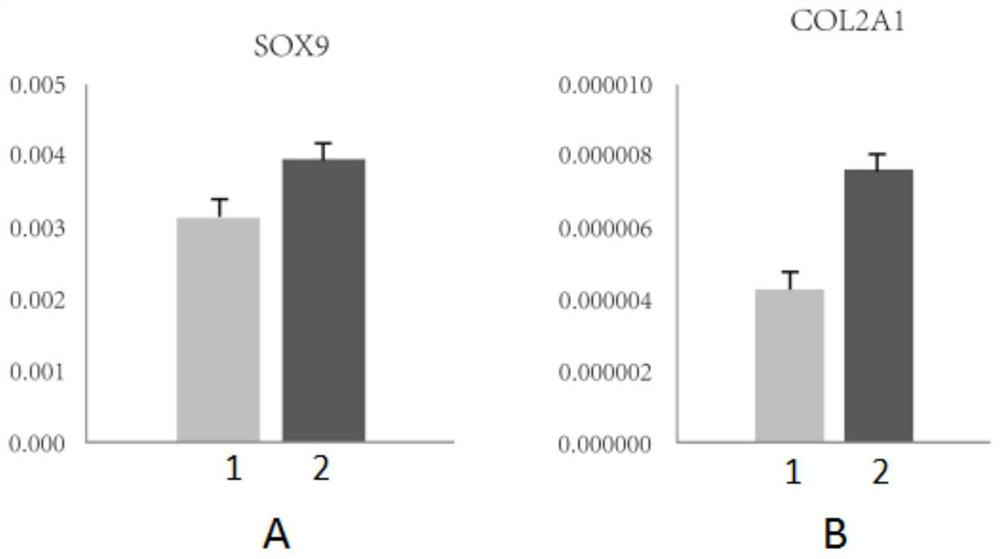
The invention discloses a cell culture solution for enhancing cartilage differentiation induction, a method and an application. A blood platelet lysate is extracted and prepared from clinically wasteplacental blood, so that the source is wide, and the cost is low. Mesenchymal stem cells obtained by the method can stably grow in an adherent manner, characteristics are similar to those of cells obtained by a conventional culture method, and the cells are in a typical fusiform vortex shape under a microscope; and compared with the conventional culture method, the method has the advantages that expression of cartilage differentiation marker genes SOX9 and COL2A1 in the cells can be effectively activated, the number and strength of formed cartilages after in-vitro induction are obviously improved, and the higher chondroblast differentiation capacity is shown.
Owner:北京中卫医正科技有限公司
Method for preparing high-activity exosome through heterologous serum-free 3D culture of MSC stem cells
ActiveCN112760293AIncrease productionImprove expression levelPeptide/protein ingredientsNGF/TNF-superfamilyDrug carrierExosome
The invention belongs to the field of natural biological nano materials, and particularly relates to an HPL culture medium for culturing MSC stem cells and a method for preparing a high-activity exosome by heterologous serum-free 3D culture of the MSC stem cells. According to the HPL culture medium for culturing the MSC stem cells, provided by the invention, human-derived platelet lysate is used for replacing animal serum FBS for cell culture. The method for preparing the high-activity exosome through heterologous serum-free 3D culture of the MSC stem cells is simple in preparation process, efficient and low in cost. The yield of the exosome is much higher than that of a conventional 2D cell culture method, the prepared exosome is higher in anti-cancer activity and safer to use, and a good foundation is laid for potential clinical application of MSC stem cell-derived biological nano-drug carrier treatment on cancers and the like.
Owner:GUANGDONG UNIV OF TECH
Platelet lysate supported micro-sphere preparation method
ActiveCN108715833AGood biocompatibilityEffective proliferationSkeletal/connective tissue cellsBlood/immune system cellsDouble-timeMicrosphere
The invention relates to a platelet lysate supported micro-sphere preparation method, and aims to solve the problem of large platelet lysate consumption or poor micro-carrier adsorption effect in existing cell culture. The preparation method includes the steps of platelet lysate preparation, micro-sphere component preparation and platelet factor and micro-capsule supported micro-sphere preparation. A prepared micro-sphere is quite strong in osteoblast carrying capacity, most of cells are fitted on the micro-sphere under the condition that the ratio of osteoblasts to the micro-sphere is 500:1,and only few individual cells are scattered in culture solution. Mesenchymal stem cells on the micro-sphere are good in growth condition, cell viability exceeds 95%, doubling time is short, positive expression rate exceeds 95% in terms of purity, and the preparation method is applied to the technical field of biology.
Owner:天晴干细胞股份有限公司
Injection-type platelet lysate-loaded temperature-sensitive hydrogel-high-dispersion nanoparticle system and application thereof
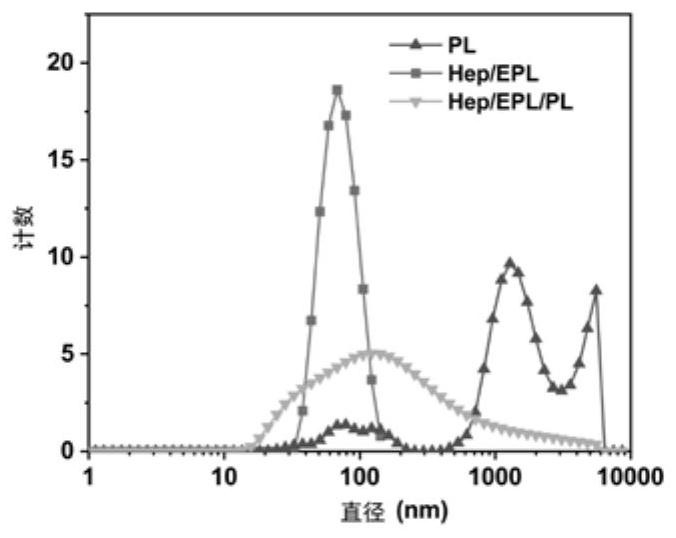
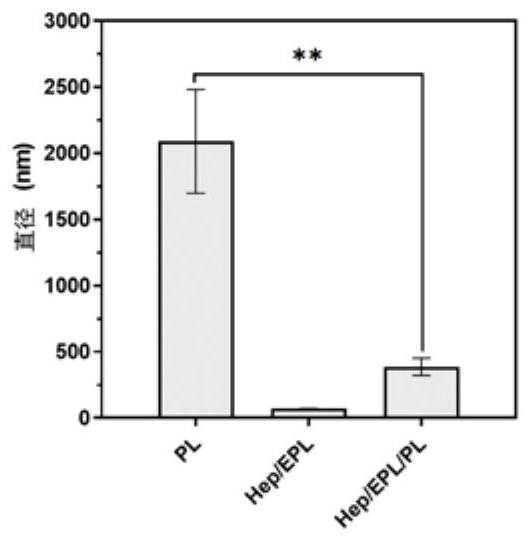
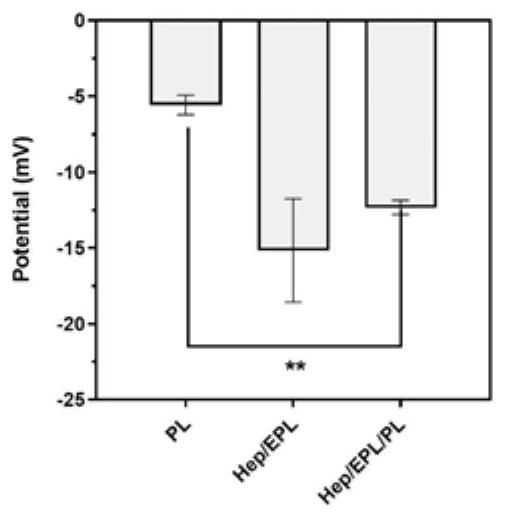
PendingCN112107733AReasonable materialsPharmaceutical delivery mechanismTissue regenerationMicrosphereNanoparticle
The invention relates to an injection-type platelet lysate-loaded temperature-sensitive hydrogel-high-dispersion nanoparticle system. The system comprises temperature-sensitive hydrogel, and a platelet lysate-loaded heparin / epsilon-polylysine nanoparticle embedded into the temperature-sensitive hydrogel. A double slow-release microsphere hydrogel system is designed for the first time to load platelet lysate, and the system is applied to cartilage tissue engineering. The effects of single injection, long-acting slow release and continuous curative effects are achieved. A material is designed according to the characteristics of the platelet lysate itself for the first time, the situation that the platelet lysate with heterogeneous particle distribution can affect the physical characteristicsof the hydrogel is found for the first time, and the situation can be well improved through microsphere construction, so that the constructed material is more reasonable.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Method for culturing autologous umbilical cord mesenchymal stem cells with platelet-rich lysate from human umbilical cord blood
ActiveCN103352026BDoes not affect storageNormal colonySkeletal/connective tissue cellsBlood/immune system cellsFactor iiUmbilical cord
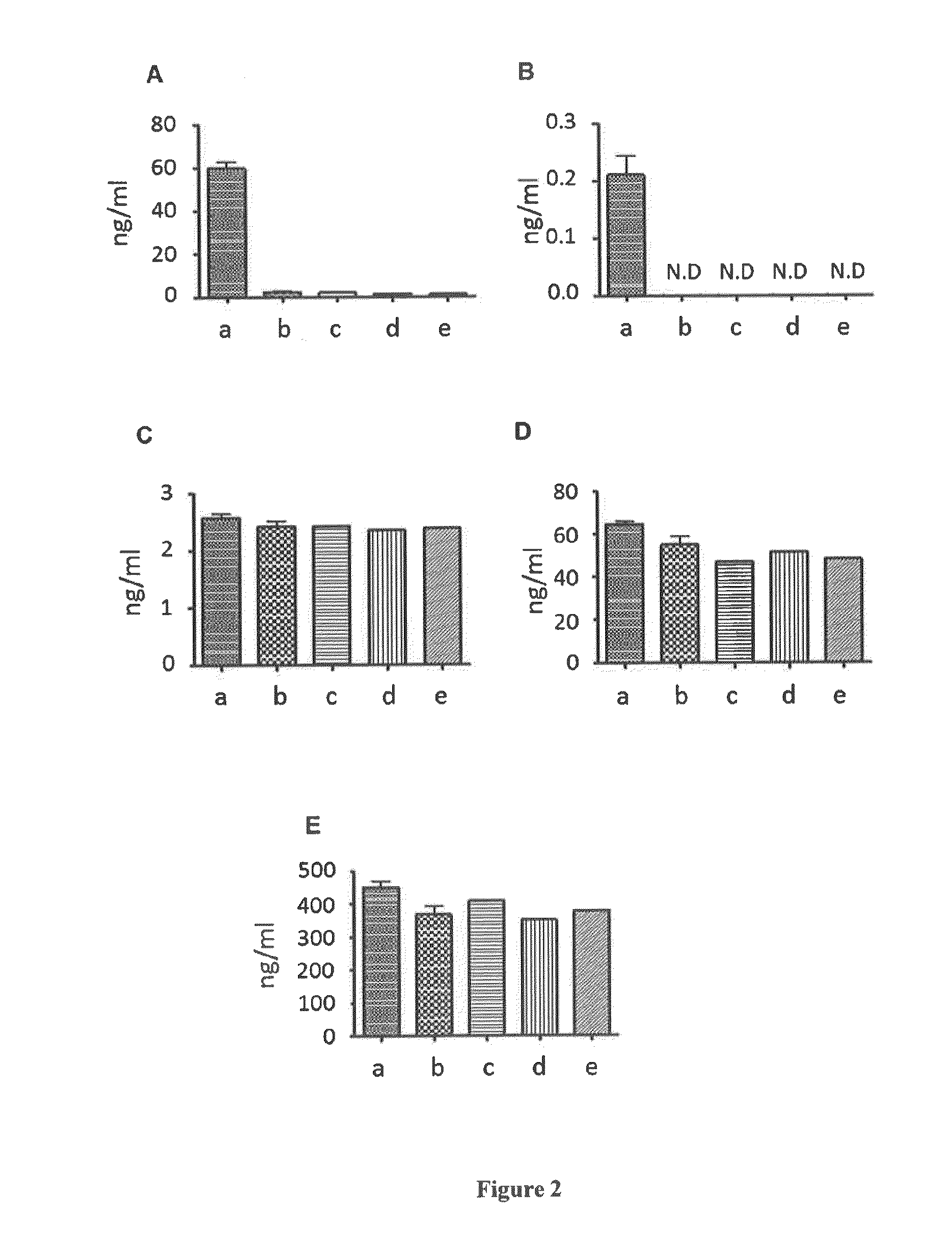
The invention relates to a method for cultivating autologous umbilical cord mesenchymal stem cells by adopting human umbilical cord blood rich platelet lysate. The conventional cell bank preparing processes adopt blood serum of calves or fetal calves to cultivate, digest, cryopreserve and the like, so that the risk of heterogeneous protein residue certainly exists in the application of clinic umbilical cord stem cells in the future. The method comprises the following steps of umbilical cord blood rich platelet serum preparation, platelet lysate preparation, preparation of serous without platelet, the confirmation of the content of cell factors, namely PDGF-AB, FGF2, TGF-Beta and VFGF in the platelet lysate, the separation and the primary culture of umbilical cord stem cells, the culture and passage of umbilical cord stem cells, the cryopreservation of umbilical cord stem cells, and the karyotype analysis. The method is used for cultivating stem cells.
Owner:武汉天晴干细胞有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com