Platelet Lysate and Bioadhesive Compositions Thereof for the Treatment of Mucositis
a technology of platelet lysate and composition, applied in the field of medicine, can solve the problems of no entirely satisfactory therapeutic agent, no therapeutic agent at all, and difficult to reach or subject to removal mechanisms of therapeutic agents,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of composition 1
[0084]a) Preparation of the Carrier
[0085]A gel based upon polyacrylic acid (carbopol 974-P NF) is prepared having the following composition:[0086]carbopol 5% (p / p)[0087]saccharin 0.2% (p / p)[0088]flavourings 0.2% (p / p)[0089]NaOH4N up to pH 7.
[0090]In particular, the saccharin is dissolved in a physiological solution (0.9% weight / volume of NaCl) through magnetic agitation. Carbopol is added and the mixture is kept under agitation until complete hydration (about 12 hours). When hydration is complete, a flavouring is added, for example mint, liquorice or strawberry. The formulation is then buffered to pH 7 through the use of a solution of NaOH4N in order to obtain the gelification of the carbopol and thus a formulation having a pH compatible with the mucosa of the oral cavity.
[0091]The carrier thus prepared is then poured into a heat-resistant glass bottle, the empty space at the top is filled with nitrogen and it is subjected to sterilisation with damp heat ...
example 2
Preparation of Composition 2
[0094]a) Preparation of the Carrier for Composition 2
[0095]A gel based upon chitosan and hydroxypropylmethylcellulose (HPMC) was prepared with the following composition:[0096]chitosan glutamate 6% (p / p)[0097]HPMC 2% (p / p)
[0098]The hydroxypropylmethylcellulose is hydrated with a minimum amount of distilled water.
[0099]Chitosan glutamate is added until a concentration of 6% p / p is obtained and the whole thing is brought up to volume with water and kept under mild agitation until the complete hydration of the polymers.
[0100]The pH of the carrier thus prepared is equal to 5.5.
[0101]The gel is then poured into a heat-resistant glass bottle, the empty space at the top is filled with nitrogen and it is subjected to sterilisation with damp heat in an autoclave for 15 minutes at 121° C.
[0102]b) Preparation of Composition 2
[0103]The carrier obtained in step a) is then mixed with platelet lysate in a 1:1 weight ratio up to a final concentration of chitosan glutamate...
example 3
Characteristics of Composition 1
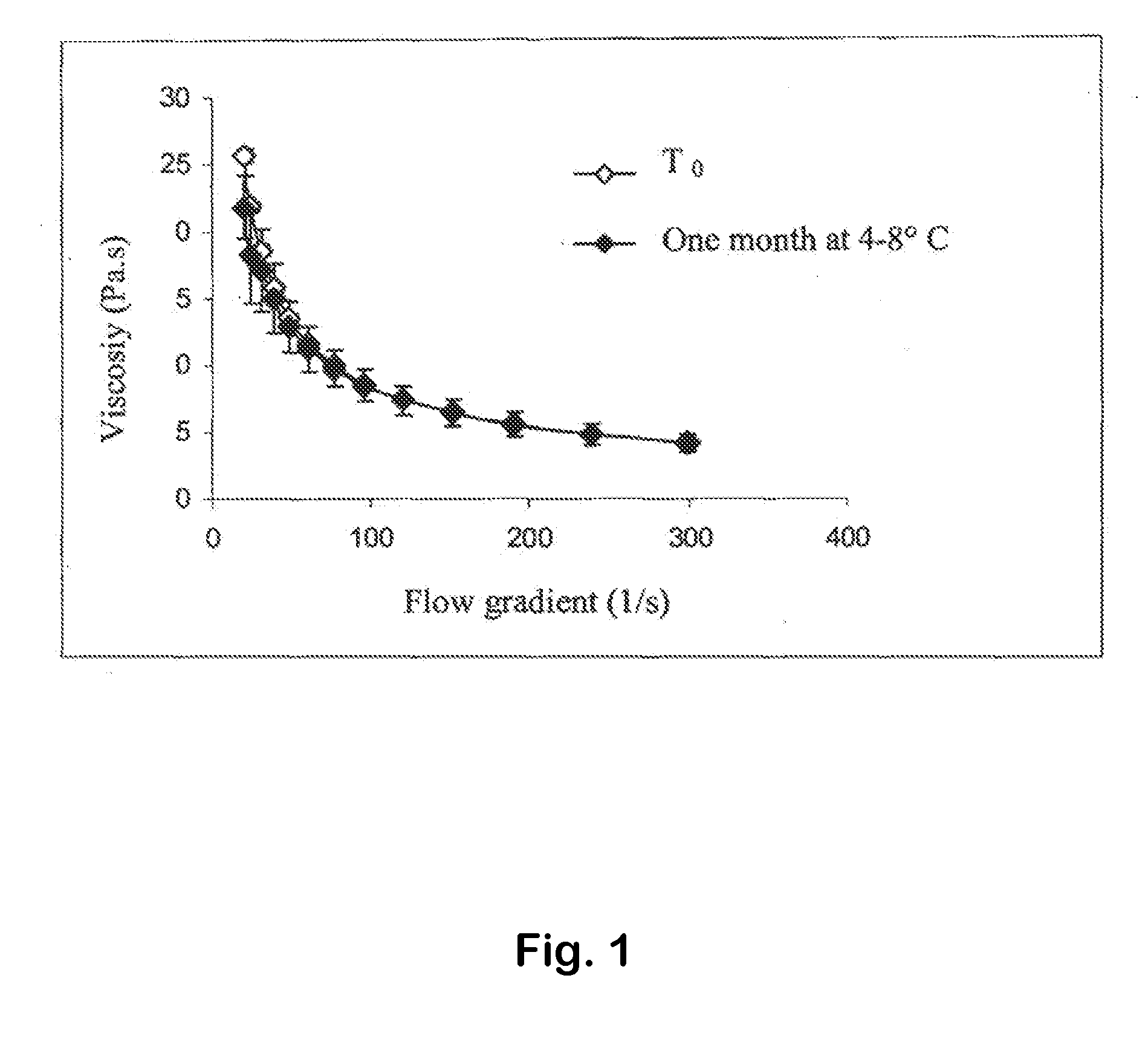
[0104]The composition 1 has a pH value equal to about 7 and rheological properties suitable for application in the oral cavity: apparent viscosity at 50 s−1 equal to about 4.5 Pa·s.
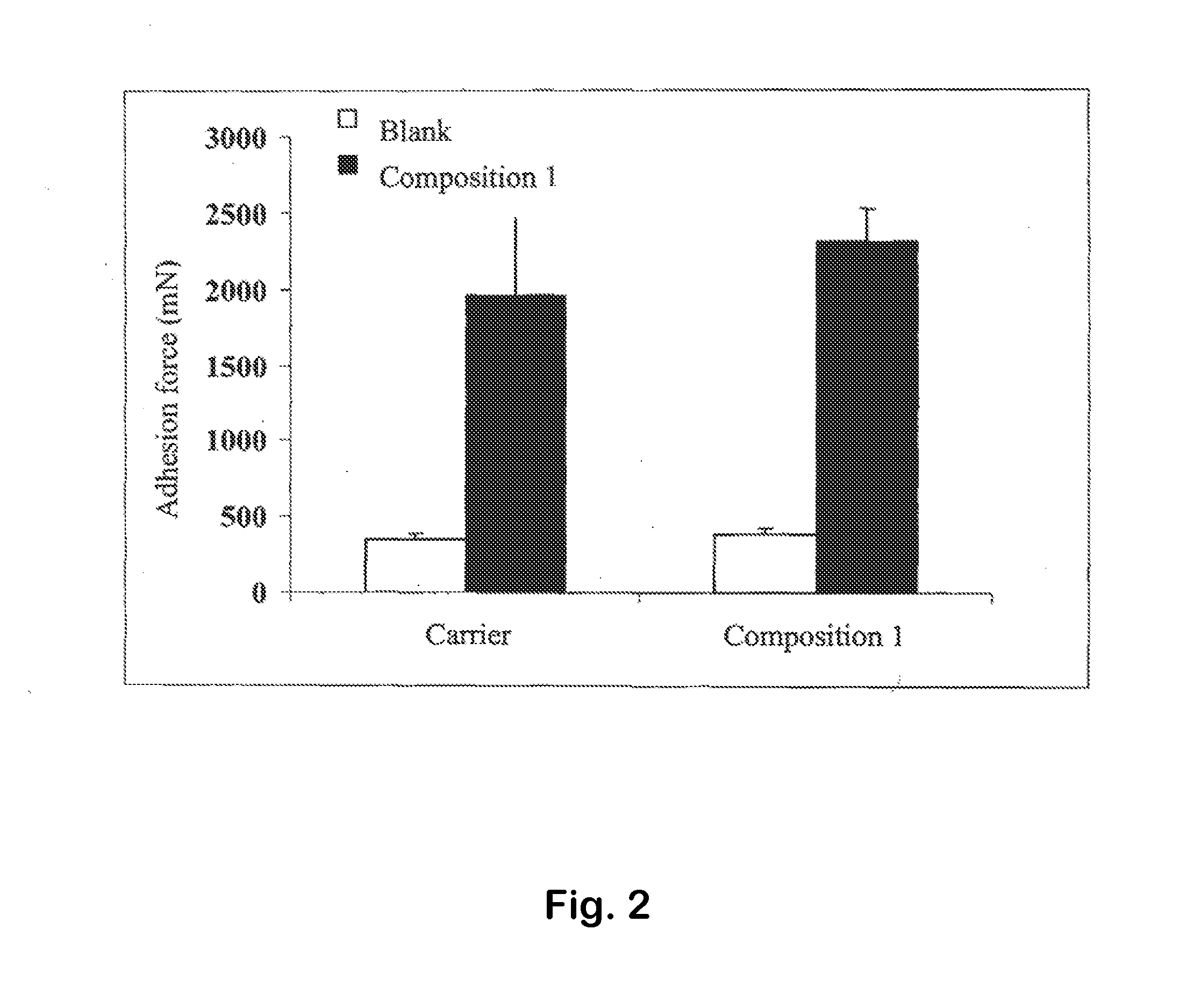
[0105]The composition has properties of mucoadhesion as illustrated in FIG. 2.
[0106]The composition maintains the ability to promote cellular proliferation even after conservation at a temperature of 4-8° C. for 10-15 days as represented in FIG. 4.
[0107]The composition 1 in a preliminary in vivo study proved effective in the treatment of mucositis in five patients suffering from mucositis of the oral cavity of grade III-IV.
[0108]In particular, in 4 such patients the response involved a recovery of the integrity of the damaged of between 50% and 100% and only in one case was it less than 50%. In just one patient with grade I mucositis was there no response.
[0109]No patients suffered local infections as a result of application of the lysate in mucoadhesive carrier.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com