Ophthalmic composition
a technology of ophthalmic composition and composition, which is applied in the field of ophthalmic composition, can solve the problems of further aggravating the corneal lesions, unsatisfactory recovery, and the inability to achieve any of the above-mentioned treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experimental example 1
Influence on the Repair Process of the Wound of Keratoepithelial Detachment in Alloxan-induced Diabetic Rabbits
[0063] 1) Test Animals and Procedures to Induce Diabetes
[0064] Alloxan monohydrate (Lot No. DLJ5619, M.W. 160.09 Wako Pure Chemical Industries, LTD.) of 80 mg / kg was dissolved in 2 mL of physiological saline, and the solution was administered once intravenously of male Japanese albino rabbits [Std: JW / CSK] (11-week-old) to induce diabetes.
[0065] Blood glucose was determined weekly after administration of alloxan monohydrate: the mean blood glucose was increased from 142.2.+-.4.7 mg / dL (mean.+-.S.E.; The same is applicable hereinafter.) before administration to 522.5.+-.13.7 mg / dL at 1 week, and this elevated level persisted thereafter. Animals with this alloxan-induced diabetes were used in the experiment.
[0066] 2) Detachment of Keratoepithelium
[0067] Under nembutal anesthesia a circular filter paper of TOYO No. 2 7.0 mm in diameter permeated with 7 .mu.L of n-heptanol was ...
experimental example 2
Influence on the Repair Process of the Wound of Keratoepithelial Detachment in Normal Rabbits
[0078] In the Experimental Example 1, the influence in rabbits with alloxan-induced diabetes was investigated, while the influence in normal (not diabetic) rabbits was investigated in this Example.
[0079] 1) Test Animals
[0080] Ten-week-old male New Zealand White rabbits (weighing 2.07.+-.0.11 kg: mean weight at the start of the experiment) were acclimation period for 7 days, while the animals were examined for the general signs including diarrhea and body weight, etc., and the anterior ocular segment was also observed. Only animals without any abnormality were used in the experiment.
[0081] 2) Detachment of Keratoepithelium
[0082] Keratoepithelial detachment wounds were prepared as described in the Experimental Example 1.
[0083] 3) Measurement of Area of the Keratoepithelial Detachment Wound
[0084] The test substance was instilled every 4 hours after the keratoepithelial detachment (the first ins...
experimental example 3
Influence on the Deteriorated Corneal esthesia and Hypolacrimation in Alloxan-induced Diabetic Rabbit
[0092] 1) Test Animals and Procedure to Induced Diabetes
[0093] Alloxan monohydrate (Lot No. DLJ5619, M.W. 160.09 Wako Pure Chemical Industries, LTD.) of 80 mg / kg was dissolved in 2 mL of physiological saline, and the solution was administered once intravenously of male Japanese albino rabbits [Std: JW / CSK] (11-week-old) to induce diabetes.
[0094] Blood glucose was determined once a week on 4 times after administration of alloxan monohydrate: animals having always blood glucose of 300 mg / dL or more were used as diabetic animals in this Experiment.
[0095] 2) Method of Administration
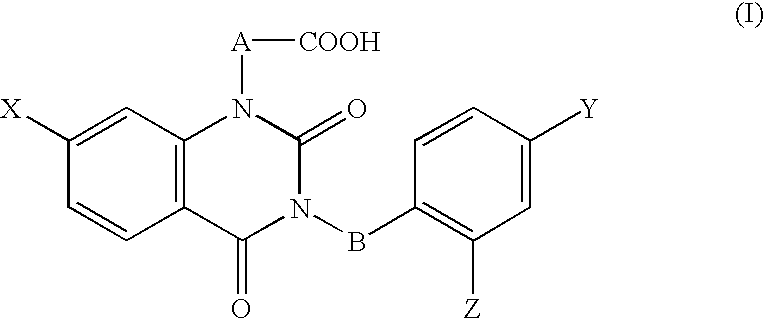
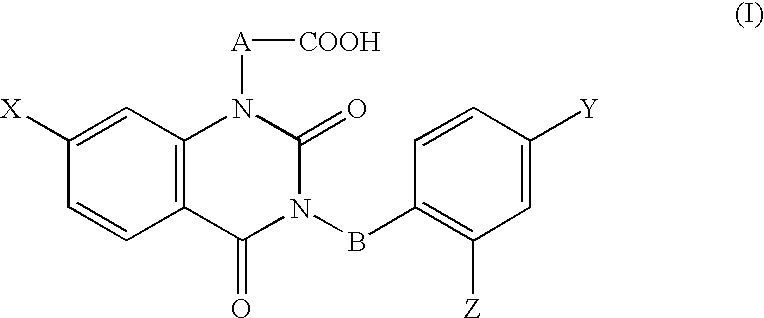
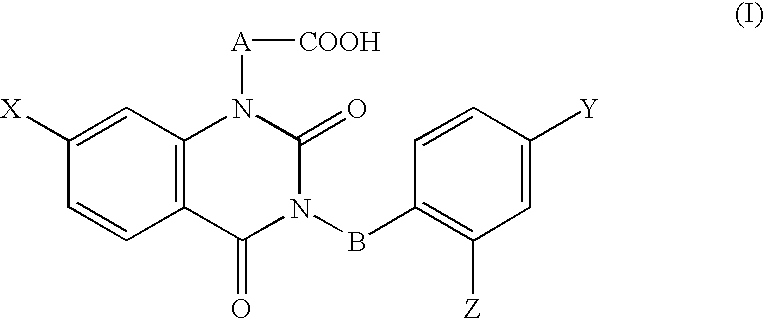
[0096] [3-(4-Bromo-2-fluorobenzyl)-7-chloro-2,4-dioxo-1,2,3,4-tetrahydroqu-inazolin-1-yl]acetate, an active ingredient of this invention, having aldose reductase-inhibiting effect was used for preparation of a 0.3% eye drops, and the eye drops were used as the test substance. The vehicle control was the vehicl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com