Culture system beneficial for mesenchymal stem cells to be applied in bone regeneration
A technology of mesenchymal stem cells and culture system, applied in cell culture active agent, culture process, tissue regeneration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
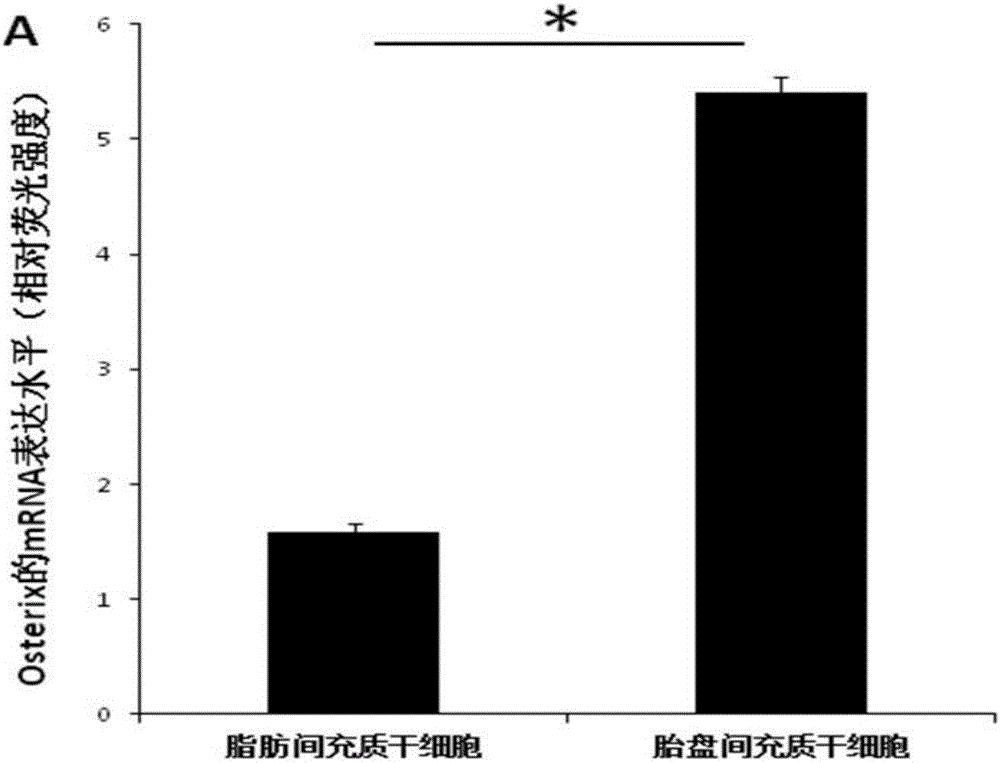
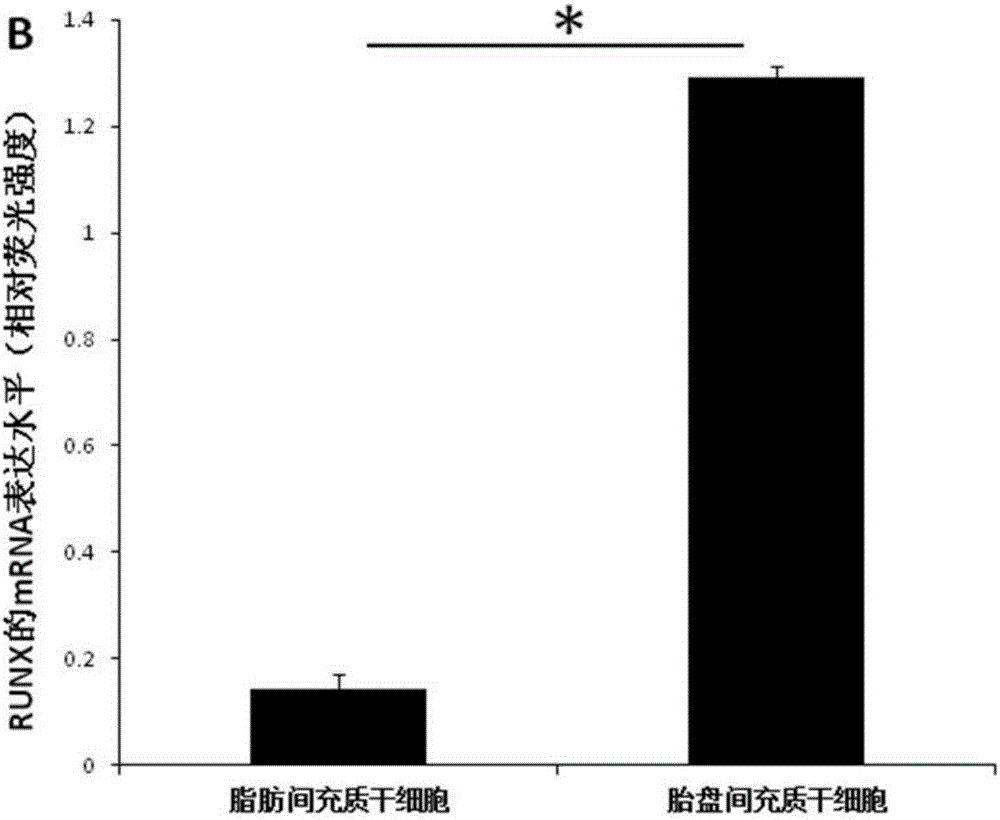
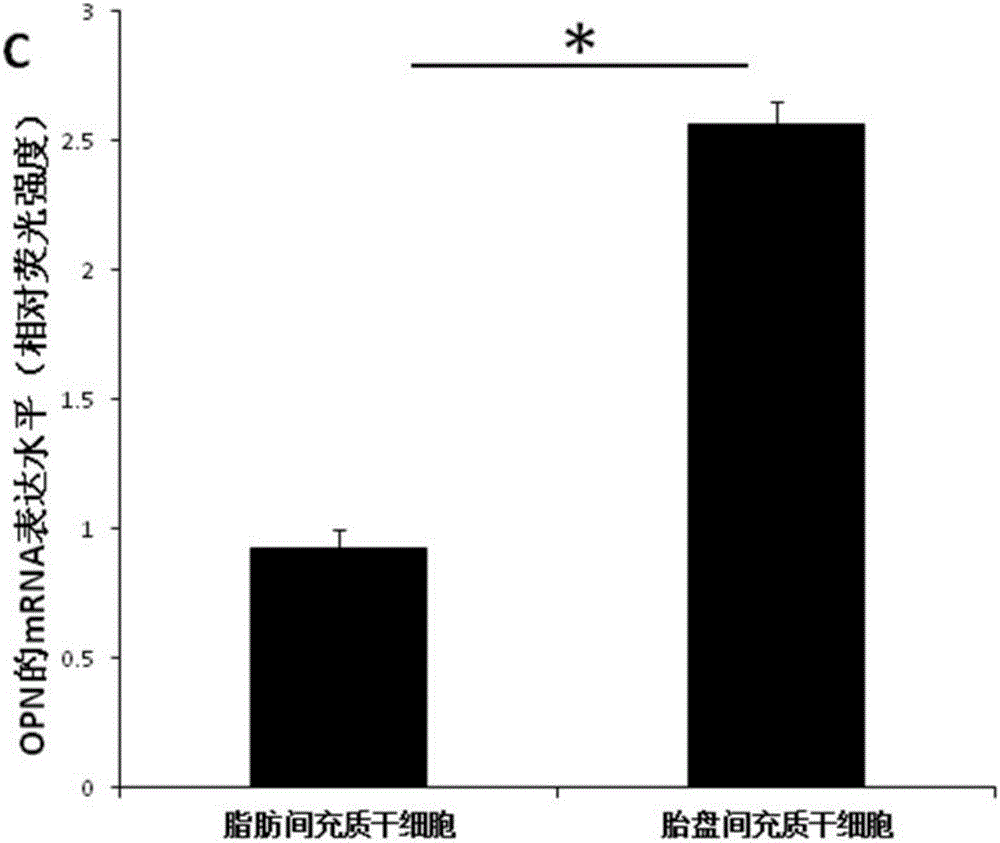
[0042] A culture system conducive to the application of placental mesenchymal stem cells in bone regeneration, the steps are as follows:
[0043] ⑴Isolation of placental mesenchymal stem cells: obtain sterile placental tissue, peel off the outer membrane of the placental tissue and the internal artery and vein of the placental tissue under aseptic conditions, separate the placental Wharton's jelly and cut it into 2-3mm 3 , paste the shredded Wharton's glue on the bottom of the culture bottle, and add an appropriate amount of culture solution to the culture bottle after 1-4 hours. The culture solution uses a-MEM+5% platelet lysate+50mg / ml ascorbic acid+10nmol / L EGF +10nmol / L bFGF+10nmol / L recombinant human brain natriuretic peptide (hBNP);
[0044] (2) Digestion and passage of placental mesenchymal stem cells. When the placental mesenchymal stem cells grow to 80%, they are digested with 0.05% trypsin + 0.02% EDTA, and 1×10 4 cells / cm 2 Transfer to a new culture bottle;
[00...
Embodiment 2
[0050] A culture system conducive to the application of umbilical cord mesenchymal stem cells to bone regeneration, the steps are as follows:
[0051] (1) Separation of umbilical cord mesenchymal stem cells: obtain sterile umbilical cord tissue, peel off the outer membrane of the umbilical cord tissue and the internal artery and vein of the umbilical cord tissue under aseptic conditions, separate the umbilical cord Wharton's jelly and cut it into 2-3mm 3 , paste the shredded Wharton's glue on the bottom of the culture bottle, and add an appropriate amount of culture solution to the culture bottle after 1-4 hours. The culture solution uses a-MEM+5% platelet lysate+50mg / ml ascorbic acid+10nmol / L EGF +10nmol / L bFGF+10nmol / L recombinant human brain natriuretic peptide (hBNP);
[0052] (2) Digestion and passage of umbilical cord mesenchymal stem cells. When the umbilical cord mesenchymal stem cells grow to 80%, digest them with 0.05% trypsin + 0.02% EDTA, and dilute them with 1×10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com