Minigene
a technology of minigene and adipose tissue, applied in the field of minigene, can solve the problem of elusive provision of optimal minigenes that maximize epitope-specific immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Epitope Identification by EI Suite
[0130]In order to identify epitope candidates that may elicit maximum tumor-specific immune response in a tolerized setting, while minimizing potential for off-target autoimmune activity, the entire CEA protein was scanned with several independent filters, using a proprietary software package called EI Suite (as described in WO 2006 / 124408). EI Suite ranks protein fragments based on binding affinity for a Class I MHC allele (in this case, HLA-A*0201), similarity to fragments of other human and murine proteins, and amenability to immunogenic enhancement.
[0131]A total of 12 HLA-A*0201-restricted peptides and peptide analogs were identified by EI Suite as having the highest potential for use in an epitope-based vaccine or for in vitro monitoring of vaccine-induced CEA-specific CTL responses. The selected CEA peptide epitopes are shown in Table 1
SEQIDPositionPeptideNO.TypeCEA.691IMIGVLVGV 1Wild typeCEA.411V10VLYGPDDPTV 2Anchor-modified analogCEA.690L2GL...
example 2
The A*0201 Binding Affinity Filter
[0132]HLA binding affinity has been shown to correlate with T-cell recognition for peptides derived from viral antigens (Sette et al. J. Immunol. 153: 5586-92 (1994)). More recently, this relationship has also been observed for tumor epitopes (Keogh et al. J. Immunol. 167: 787-796 (2001)). Selecting potential epitopes on the basis of HLA binding affinity is, therefore, an efficient alternative to large-scale epitope mapping or other T cell-dependent strategies.
[0133]Therefore, using EI Suite, the sequence of the CEA protein was scanned for peptides that were predicted to be strong binders to the HLA class I allele A*0201 (referred to as A2.1 below). A total of 1267 peptides representing all possible 9- and 10-mer frames of the 702 amino acid protein were evaluated. Each peptide was scored based on the degree of adherence to a statistical motif inferred from several publicly available epitope databases, including: SYFPEITHI (Rammensee et al. Immunoge...
example 3
Comparison of CEA Peptides with Fragments of Other Human Proteins
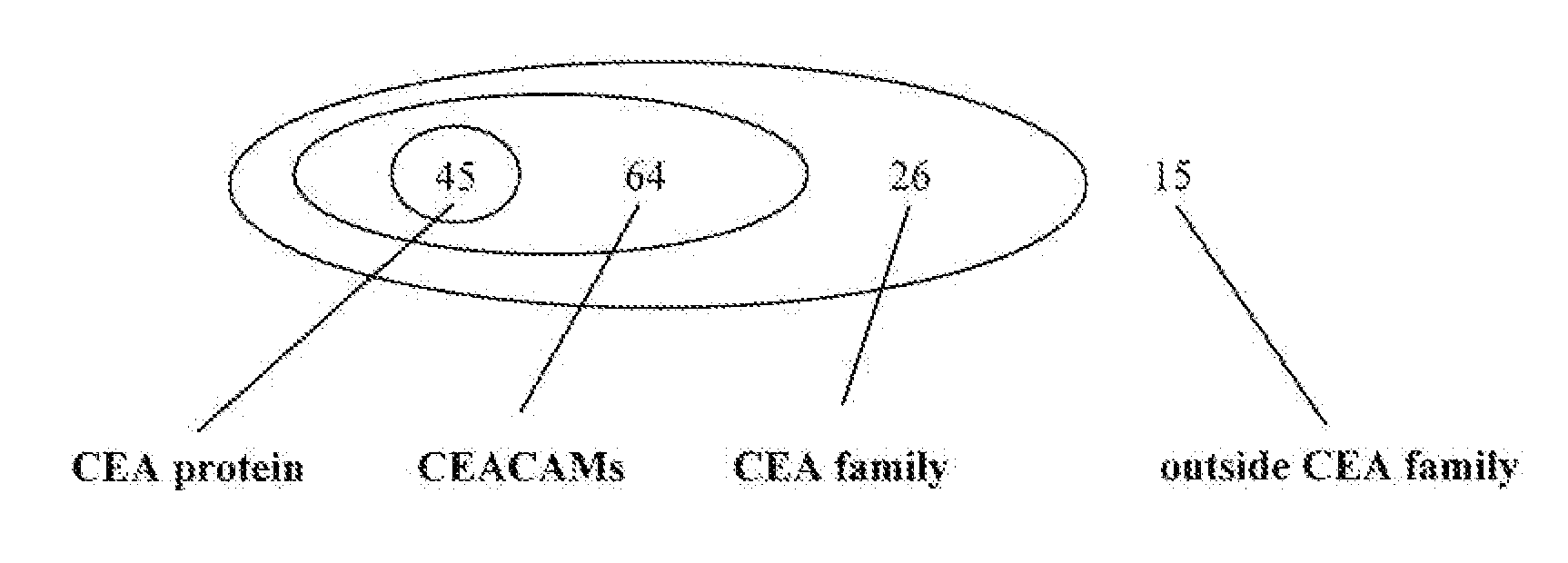
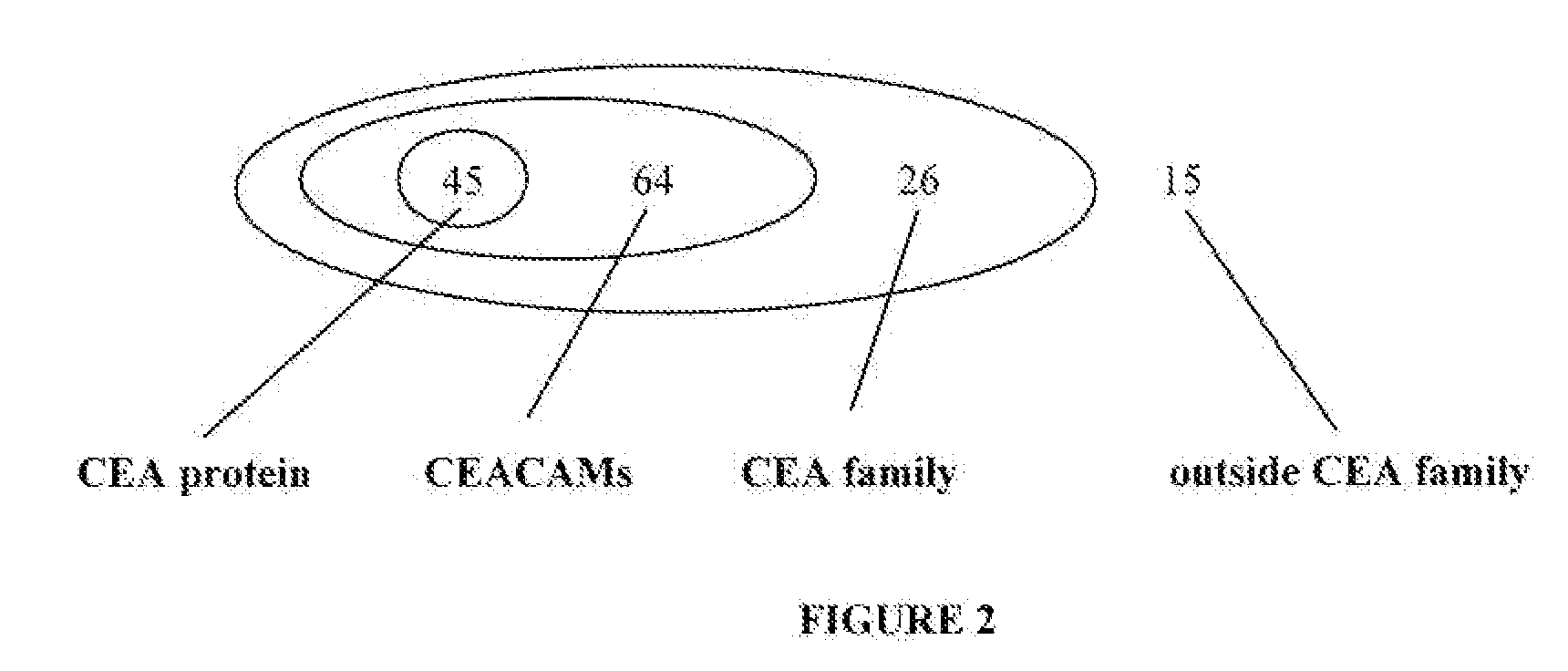
[0134]A vaccine containing only CEA-specific epitopes may prevent the off-target T-cell response that sometimes occurs when immune tolerance is broken to a shared antigen. The CTL-mediated destruction of melanocytes (van Elsas et al. J. Exp. Med. 190: 355-366 (1999)) and pancreatic islet β-cells (Ludewig et al. J. Exp. Med. 2000, 191: 795-804 (2000)) following immunotherapy are two examples of such off-target immune response. Moreover, CEA peptides that are also found in other proteins that are expressed in multiple normal tissues are more likely to have been presented to T-cells in a tolerizing setting Immune tolerance to such peptides may therefore be more difficult to overcome. For this reason, EI Suite was used to select, from the 150 top-scoring CEA peptides, those unique to the CEA protein.
[0135]It is known that T-cells can often recognize cognate epitopes comprising modifications at the HLA contact positions (po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com