Tazarotene derivatives
a technology of tazarotene and derivatives, which is applied in the field of derivatives of tazarotene, can solve the problems of difficult formulation chemists, retinoids and antibiotics are often readily degraded,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Degradation of Tazarotene in the Presence of Benzoyl Peroxide
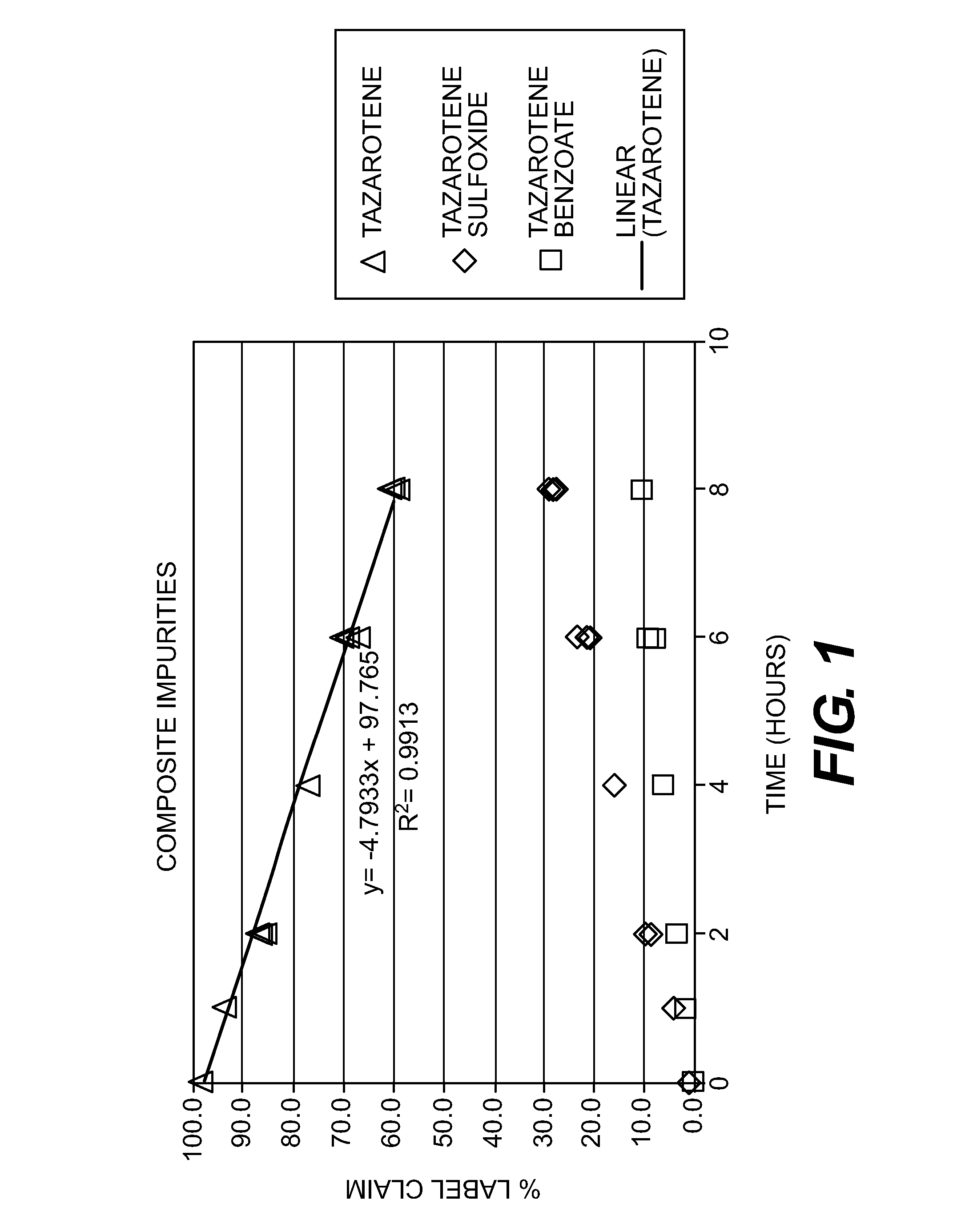
[0177]DUAC® gel (1% clindamycin and 5% benzoyl peroxide marketed by Stiefel Laboratories, Inc.) and TAZORAC® cream (0.1% tazarotene marketed by Allergan, Inc.) have been successfully used to treat facial acne. However, these topical treatments are not approved for concomitant use. To study whether tazarotene is susceptible to oxidative decomposition by benzoyl peroxide, an in vitro laboratory study was conducted wherein a mixture of DUAC gel and TAZORAC cream was prepared.
[0178]Samples were prepared by taking equal portions of DUAC gel and TAZORAC cream and mixing them thoroughly at room temperature with a spatula in a suitable container to form a uniform mixture. The initial samples were analyzed immediately by HPLC. The other samples were placed into an oven at 35° C. and removed for analysis after one, two, four, six and eight hours. An allowance was made for product evaporation over the course of the study.
[0179]FIG. 1...
example 2
Further Study of Tazarotene and its Metabolites
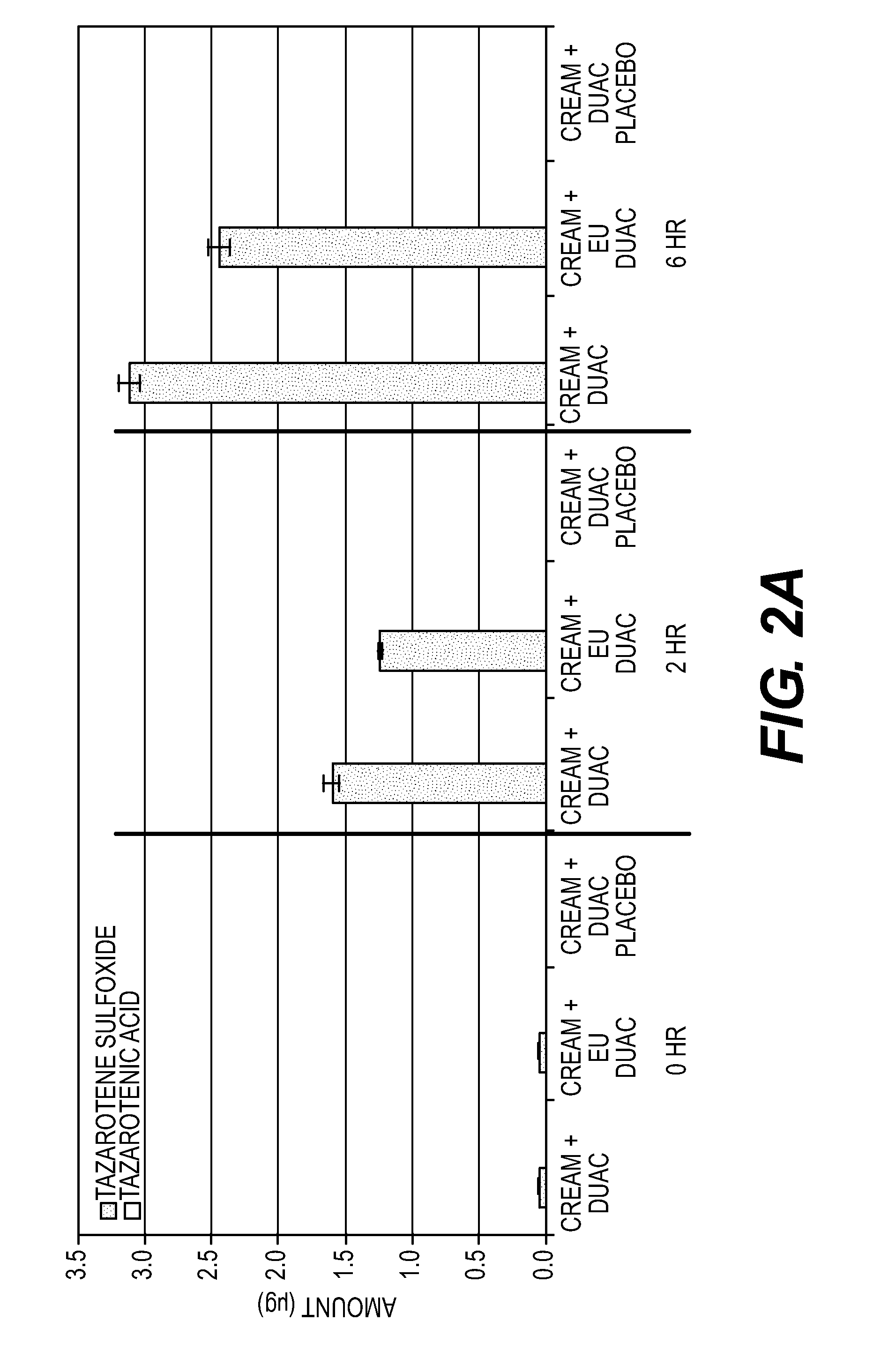
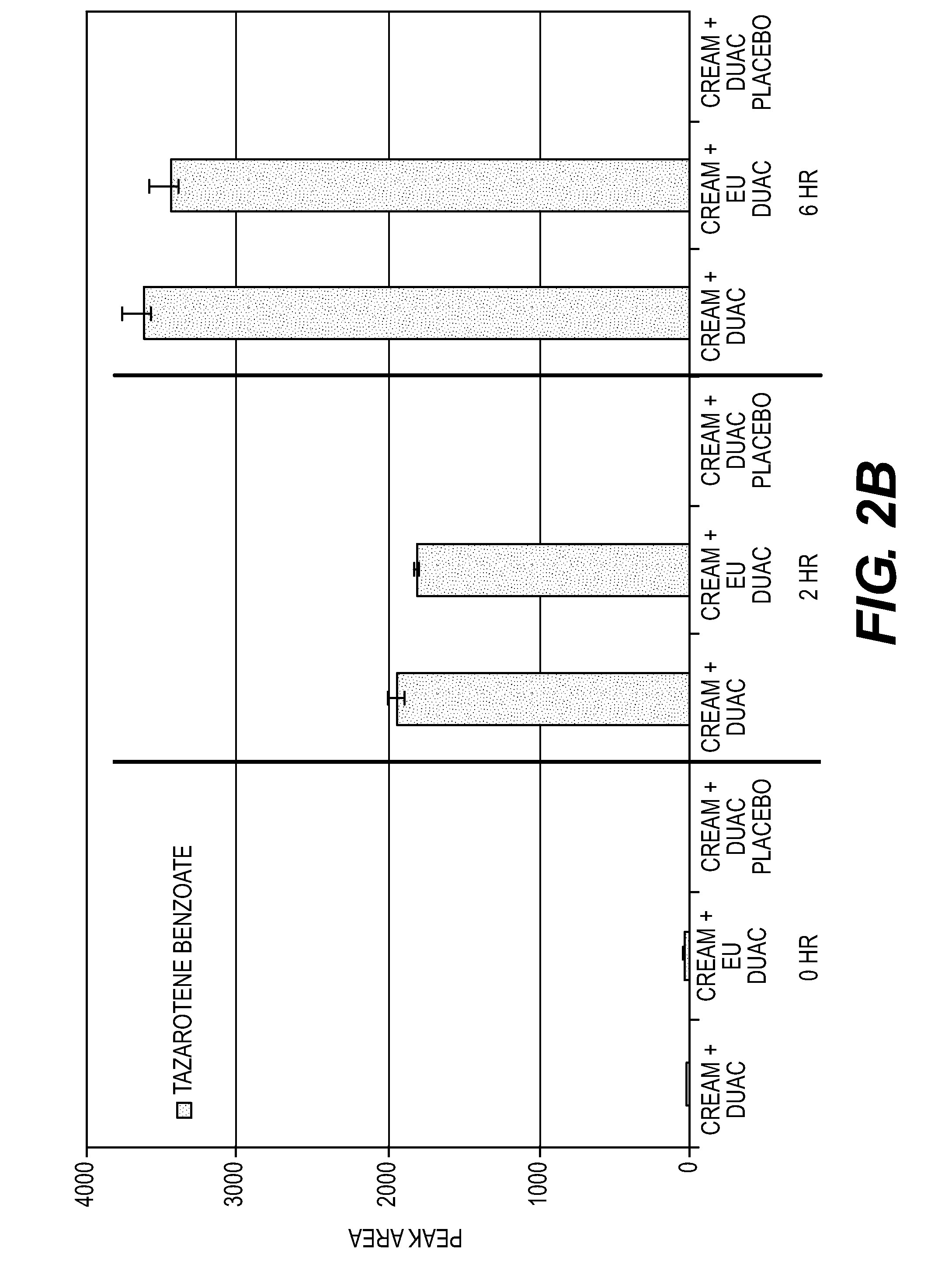
[0181]An in vitro study was conducted to assess the formation of tazarotene degradants following the application of a mixture of DUAC gel and TAZORAC cream to human skin.
[0182]Equal portions of DUAC gel and TAZORAC cream were dispensed into a glass vial and mixed for approximately three minutes with a metal spatula to ensure a homogenous mixture. Samples of European DUAC gel and US DUAC gel were used in separate experiments. The products differ inasmuch as European DUAC gel does not contain paraben preservatives. The test mixtures were then applied to the surface of split-thickness skin (˜0.25 mm) at a dose of 15.6 mg / cm2 and spread evenly using a positive displacement pipette.
[0183]After 2 and 6 hours, the skin samples were washed, tape stripped twice, and then the epidermis was peeled from the dermis using a heat block. The skin samples were then extracted with acetonitrile overnight at 4° C. The distribution of tazarotene and its deg...
example 3
Retinoid Activity of Tazarotene, Tazarotene Benzoate and Tazarotene Metabolites
[0189]A study was conducted to evaluate the retinoid activity of tazarotene, tazarotene benzoate and tazarotene metabolites (tazarotenic acid, tazarotene sulfone, tazarotenic acid sulfone and tazarotenic acid sulfoxide).
[0190]SkinEthic RHE cultures were transferred into 6-well plates containing 1.0 mL / well growth media. The cultures were equilibrated at 37° C. and the media was changed daily. The cultures were subsequently placed in 60 mm petri dishes containing 3.5 mL growth media. 6 μl aliquots of the Test Articles shown in Table 3 were applied to duplicate cultures. The cultures were incubated at 37° C. for 72 hours. At the end of the incubation period, the growth media was collected and stored at −20° C. The tissues were cut in half and one half was placed in 10% NBF for histology, while the other half was placed in RNAlater™ solution (Ambion). The following analyses were performed: a) IL-1α and IL-8 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com