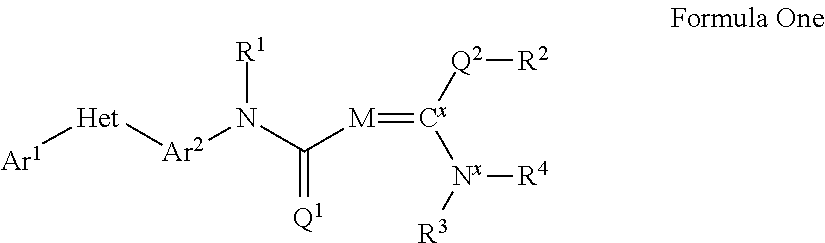
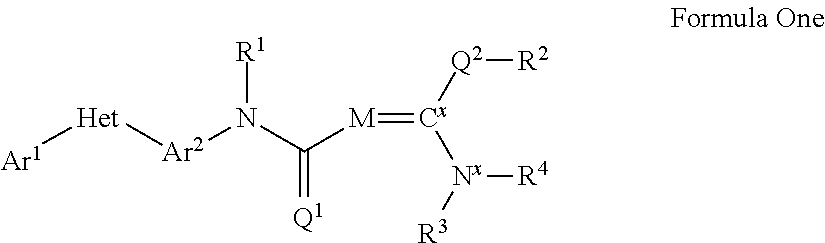
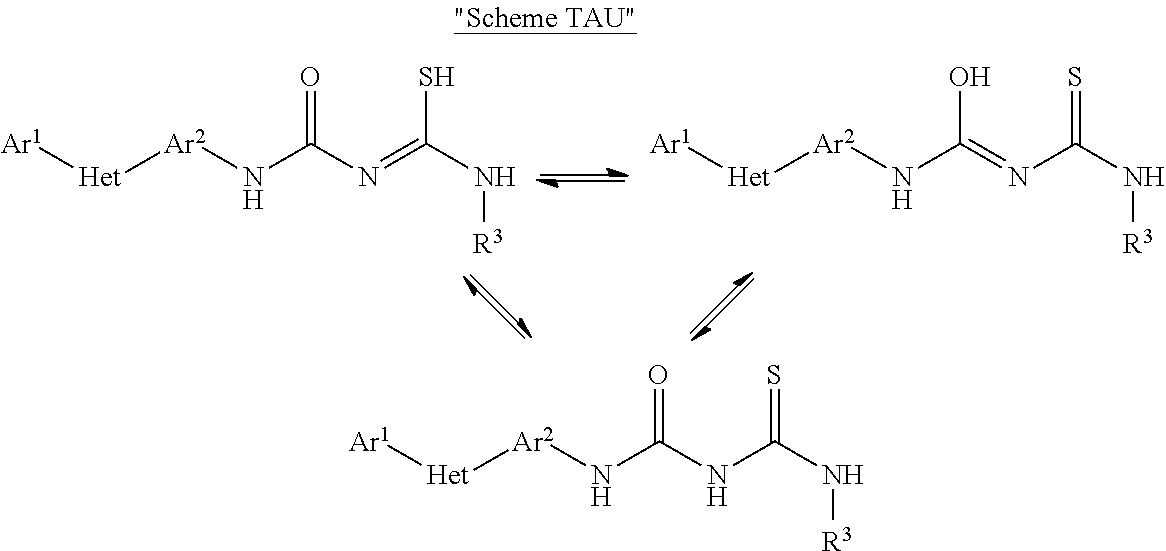
Pesticidal compositions and processes related thereto
a technology of compositions and processes, applied in the field of pesticide compositions and processes related thereto, can solve the problems of loss of agriculture, damage to all kinds of private and public structures, and millions of human deaths around the world
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (E)-((N′-(4-methoxy-2-methylphenyl)-N-((4-(1-(4-(trifluoromethyl)phenyl)-1H-1,2,4-triazol-3-yl)phenyl)carbamoyl)carbamimidoyl)thio)methyl isobutyrate (Molecule A1)
[0093]
[0094]Step 1. 2-Methyl-4-methoxyphenyl thiourea (0.5 grams (g), 2.55 millimoles (mmol)) and bromomethyl isobutyrate were combined in 5 mL of acetone at ambient temperature, and the solution was allowed to stir for 18 hours (h). The solution was then cooled to 0° C. and the resulting solid was filtered and air-dried to give (E)-(N′-(4-methoxy-2-methylphenyl)carbamimidoylthio)methyl isobutyrate HBr (B1) (0.83 g, 82%): mp 127-130° C.; 1H NMR (CDCl3) δ 11.34 (s, 1H), 10.29 (s, 1H), 8.32 (s, 1H), 7.09 (d, J=8.7 Hz, 1H), 6.79 (d, J=2.8 Hz, 1H), 6.74 (dd, J=8.7, 2.8 Hz, 1H), 3.81 (s, 3H), 2.69 (heptet, J=7.0 Hz, 1H), 2.31 (s, 3H), 1.22 (d, J=7.0 Hz, 6H); ESIMS m / z 297 ([M+H]+).
[0095]Step 2. The intermediate from Step 1 (0.40 g, 1.06 mmol) was dissolved in tetrahydrofuran (THF; 7 mL), and 4-nitrophenyl 4-(1-(4...
example 2
(Z)-Methyl N-(4-methoxy-2-methylphenyl)-N′-((4-(1-(4-(trifluoromethyl)phenyl)-1H-1,2,4-triazol-3-yl)phenyl)carbamoyl)carbamimidothioate (Molecule A2)
[0097]
[0098]The title molecule was isolated as a white solid; 38 mg (11%), mp 172-175° C.; 1H NMR (CDCl3) δ 11.29 (s, 1H), 8.64 (s, 1H), 8.17 (d, J=8.7 Hz, 2H), 7.92 (d, J=8.5 Hz, 2H), 7.80 (d, J=8.5 Hz, 2H), 7.66 (d, J=8.7 Hz, 2H), 7.33 (s, 1H), 7.16 (d, J=8.6 Hz, 1H), 6.80 (d, J=2.9 Hz, 1H), 6.75 (dd, J=8.6, 2.8 Hz, 1H), 3.82 (s, 3H), 2.38 (s, 3H), 2.30 (s, 3H); ESIMS m / z 541 ([M+H]+).
example 3
(E)-(N′-(2,6-Dimethylphenyl)-N-(4-(1-(4-(trifluoromethyl)phenyl)-1H-1,2,4-triazol-3-yl)phenylcarbamoyl)carbamimidoylthio)methyl isobutyrate (Molecule A3)
[0099]
[0100]Step 1. The intermediate (E)-(N′-(2,6-dimethylphenyl)carbamimidoylthio)methyl isobutyrate HBr (B2), was prepared from 1-(2,6-dimethylphenyl thiourea) using conditions described in Example 1. mp 129-131° C.; 1H NMR (CDCl3) δ 11.51 (s, 1H), 10.45 (s, 1H), 8.25 (s, 1H), 7.23 (d, J=7.5 Hz, 1H), 7.12 (d, J=7.4 Hz, 2H), 5.59 (s, 2H), 2.69 (heptet, J=7.0 Hz, 1H), 2.30 (s, 6H), 1.23 (d, J=7.0 Hz, 6H); ESIMS m / z 280 ([M+H]+).
[0101]Step 2. Molecule A3 was prepared in a manner similar to that described in Example 1: 575 mg (59%) of a white solid, mp 173-176° C.; 1H NMR (CDCl3) δ 11.21 (s, 1H), 8.65 (s, 1H), 8.18 (d, J=8.7 Hz, 2H), 7.92 (d, J=8.4 Hz, 2H), 7.80 (d, J=8.5 Hz, 2H), 7.68 (d, J=8.7 Hz, 2H), 7.20 (m, 1H), 7.14-7.04 (m, 2H), 5.65 (s, 2H), 2.59 (heptet, J=7.0 Hz, 1H), 2.29 (s, 6H), 1.18 (d, J=7.0 Hz, 6H); ESIMS m / z 611 ([M+...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com