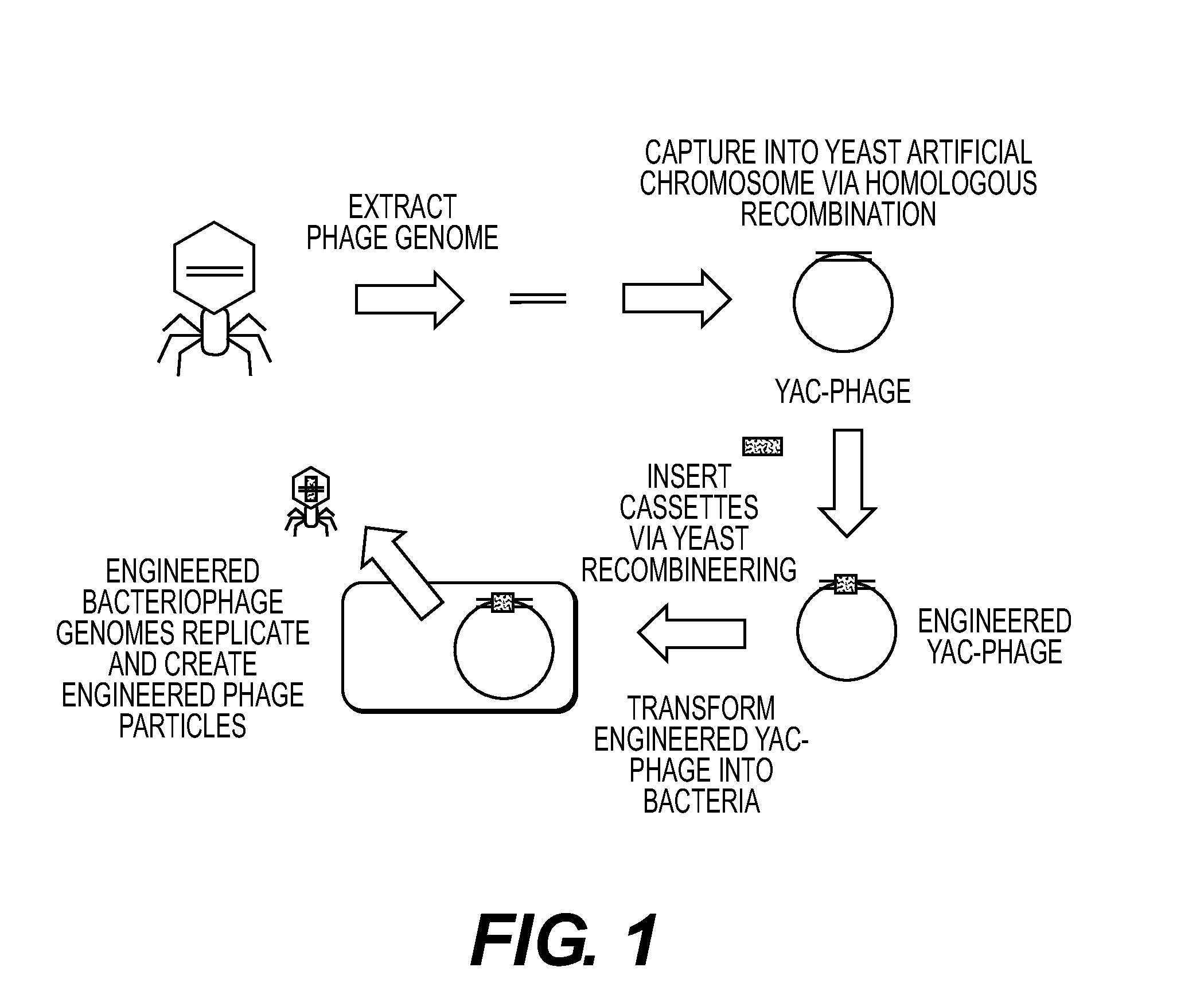
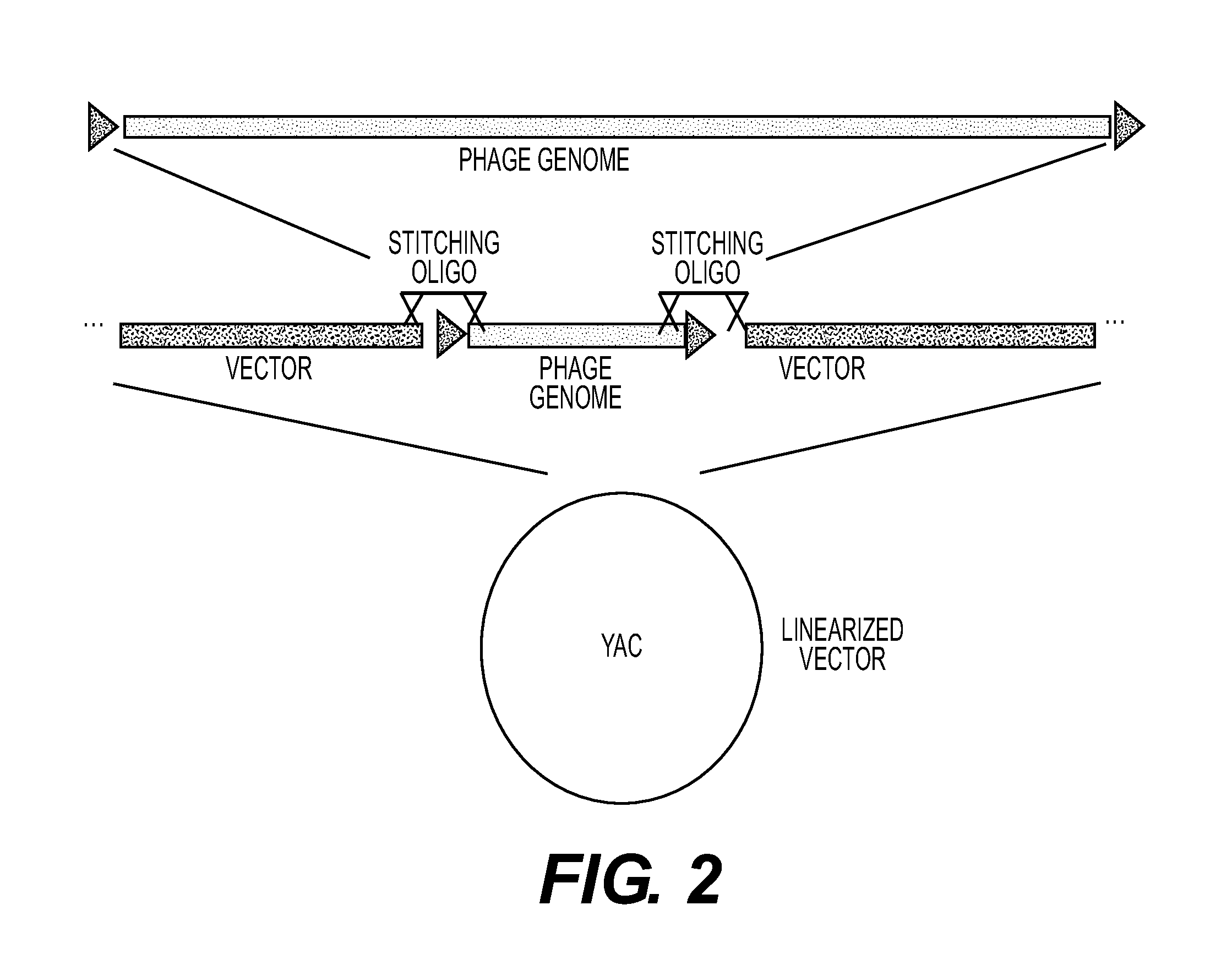
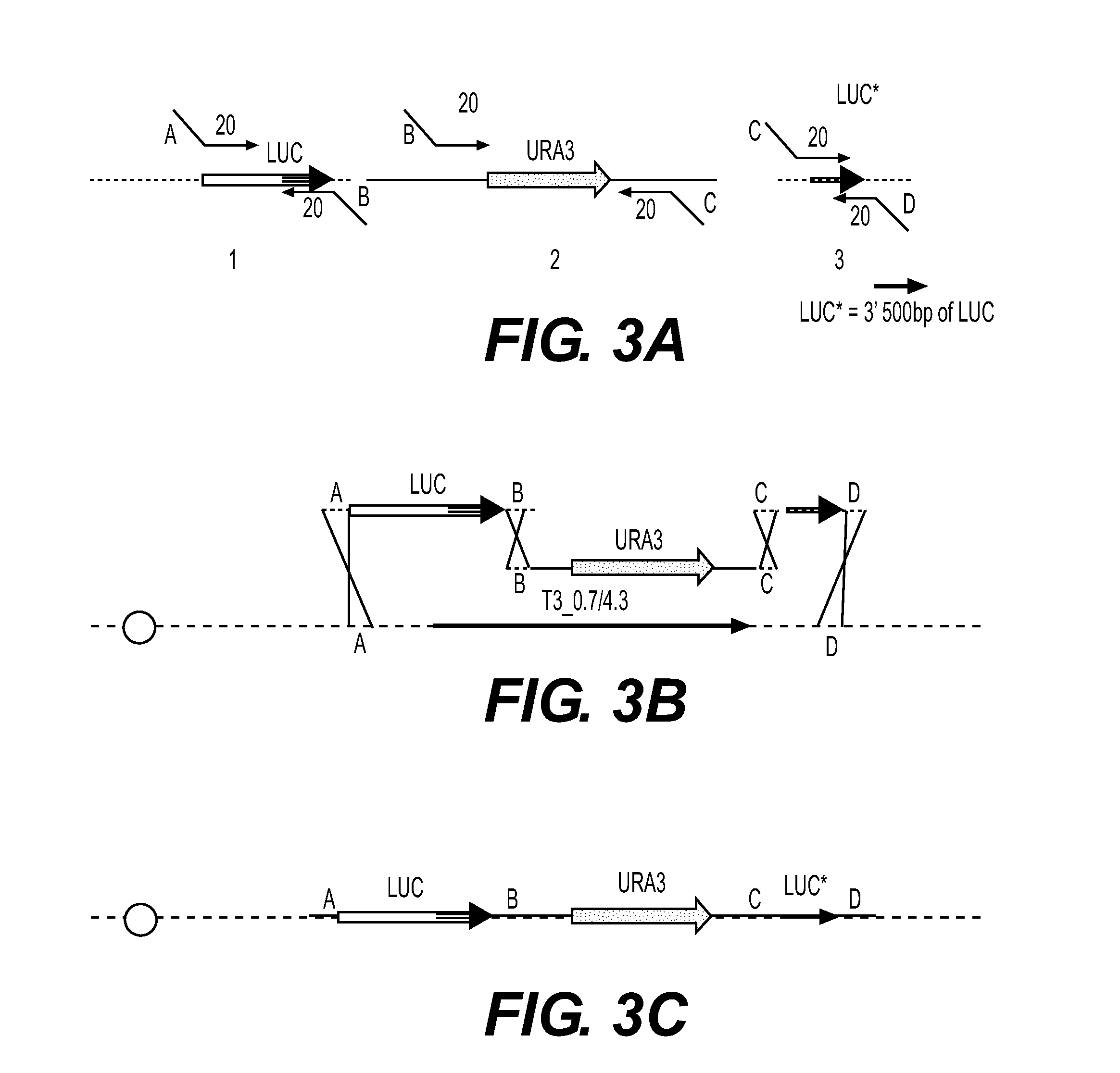
Recombinant phage and methods
a phage and phage technology, applied in the field of recombinant phage and methods, can solve the problems of limiting the natural host range of the phage engineered to date, affecting the engineering efficiency of the phage, and general lack of methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning and Genetically Modifying Phage T3
[0220]A. Phage Capture
[0221]Phage T3 was cloned and manipulated in the following manner. T3 was grown using E. coli DH10B as a host, grown in Luria Broth (LB)+2 mM calcium chloride. The phage lysate was concentrated via incubation with 10% polyethylene glycol-8000 overnight at 4° C., followed by centrifugation. The pellet was resuspended in SM buffer (Sambrook et al., Molecular Cloning: A Laboratory Manual, 3d ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (2001)). DNA was prepared from the concentrated T3 lysate using the Norgen Phage DNA kit (Cat#46700). The genomic sequence of T3 (NCBI accession #NC 003298) was used to design oligos to capture T3 into the pYES1L vector (Invitrogen®). Oligos used were duplexes of:
[SEQ ID NO: 1]CCTAGTGTACCAGTATGATAGTACATCTCTATGTGTCCCTCCTCGCCGCAGTTAATTAAAGTCAGTGAGCGAGGAAGCGCand its complement,
and duplexes of:
[SEQ ID NO: 2]GAACGACCGAGCGCAGCGGCGGCCGCGCTGATACCGCCGCTCTCATAGTTCAAGAACCCAAAGTACC...
example 2
Cloning and Genetically Modifying Phage T7
[0235]A. Phage Capture
[0236]T7 luc was created in a slightly different manner than the engineered T3 phage of Example 1.
[0237]T7 dspB (T. K. Lu and J. J. Collins, “Dispersing Biofilms with Engineered Enzymatic Bacteriophage,” Proceedings of the National Academy of Sciences, vol. 104, no. 27, pp. 11197-11202, Jul. 3, 2007, incorporated herein by reference) was captured in pYES1L by transforming genomic DNA of T7 dspB, YAC pYES1L, a duplex of:
[SEQ ID NO: 11]TTGTCTTTGGGTGTTACCTTGAGTGTCTCTCTGTGTCCCTCCTCGCCGCAGTTAATTAAAGTCAGTGAGCGAGGAAGCGC
and its complement, and a duplex of:
[SEQ ID NO: 12]CCCGAACGACCGAGCGCAGCGGCGGCCGCGCTGATACCGCCGCCGCCGGCGTCTCACAGTGTACGGACCTAAAGTTCCCCCATAGGGGGT
and its complement,
into MaV203 yeast cells (Invitrogen®). Those oligonucleotides bridge the ends of the T7 genomic sequence (NC—001604) and the YAC vector.
[0238]B. YAC to Plaque
[0239]Cloned T7 phages were shown to be able to YAC-to-plaque, as above.
[0240]Selected MaV203 cel...
example 3
Cloning and Genetically Modifying Phage T3
[0246]Phage T3 was captured into the pYES1L vector (Invitrogen®) and shown to be functional in the YAC to plaque assay as described in Example 1.
[0247]A. Luciferase and Nanoluc Insertion into T3 Phage
[0248]The T3 luciferase cassette was constructed as in Example 1.
[0249]Promega® vector pNL1.1 was the template for amplification of the nanoluc ORF with primers JHONO319 and JHONO320. pRS426 was used as a template for the Ura3 gene with primers JHNO321 and JHONO322. The sequences of those primers are:
JHONO319[SEQ ID NO: 16]AATTTACTCTTTACTCTTACAGATAACAGGACACTGAACGATGGTCTTCACACTCGAAGAJHONO320[SEQ ID NO: 17]TTACGCCAGAATGCGTTCGCACJHONO321[SEQ ID NO: 18]AGTGACCGGCTGGCGGCTGTGCGAACGCATTCTGGCGTAACAGATTGTACTGAGAGTGCACCJHONO322[SEQ ID NO: 19]ATTCAGGCCACCTCATGATGACCTGTAAGAAAAGACTCTACACACCGCATAGGGTAATAACTG.
[0250]In each case (luc cassette and nanoluc cassette), 2 PCR products were created, and co-transformed into yeast containing the T3-YAC described above ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com