Adeno-associated-virus rep sequences, vectors and viruses
a technology of vectors and viruses, applied in the field of adeno-associated virus (aav) replication (rep) sequences, can solve the problems of limited success in the prior art strategy of constructing hybrid viruses (e.g., ad/aav) by controlling rep expression, and achieve the effect of high level of rep protein expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
De Novo Synthesized Constructs:
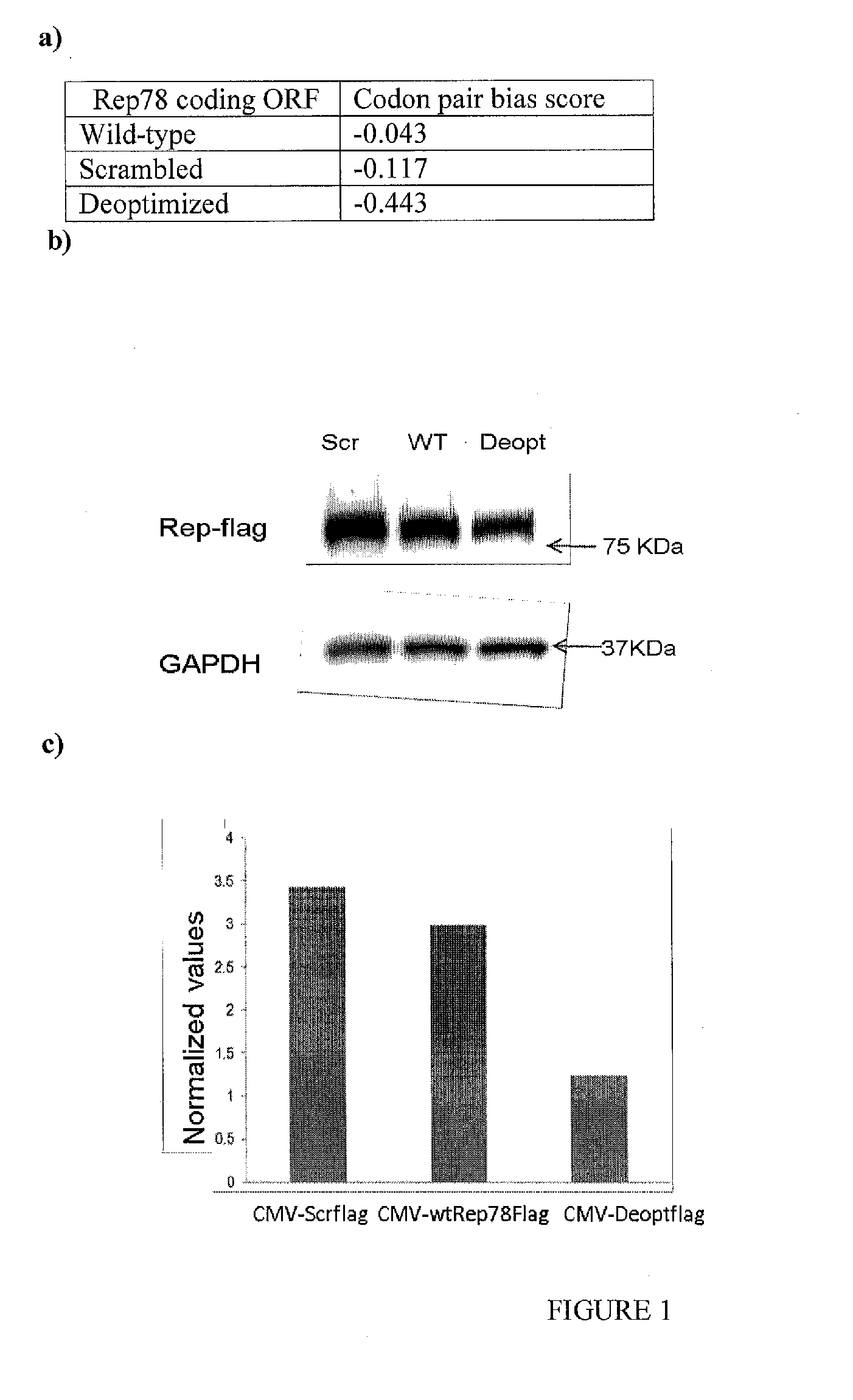
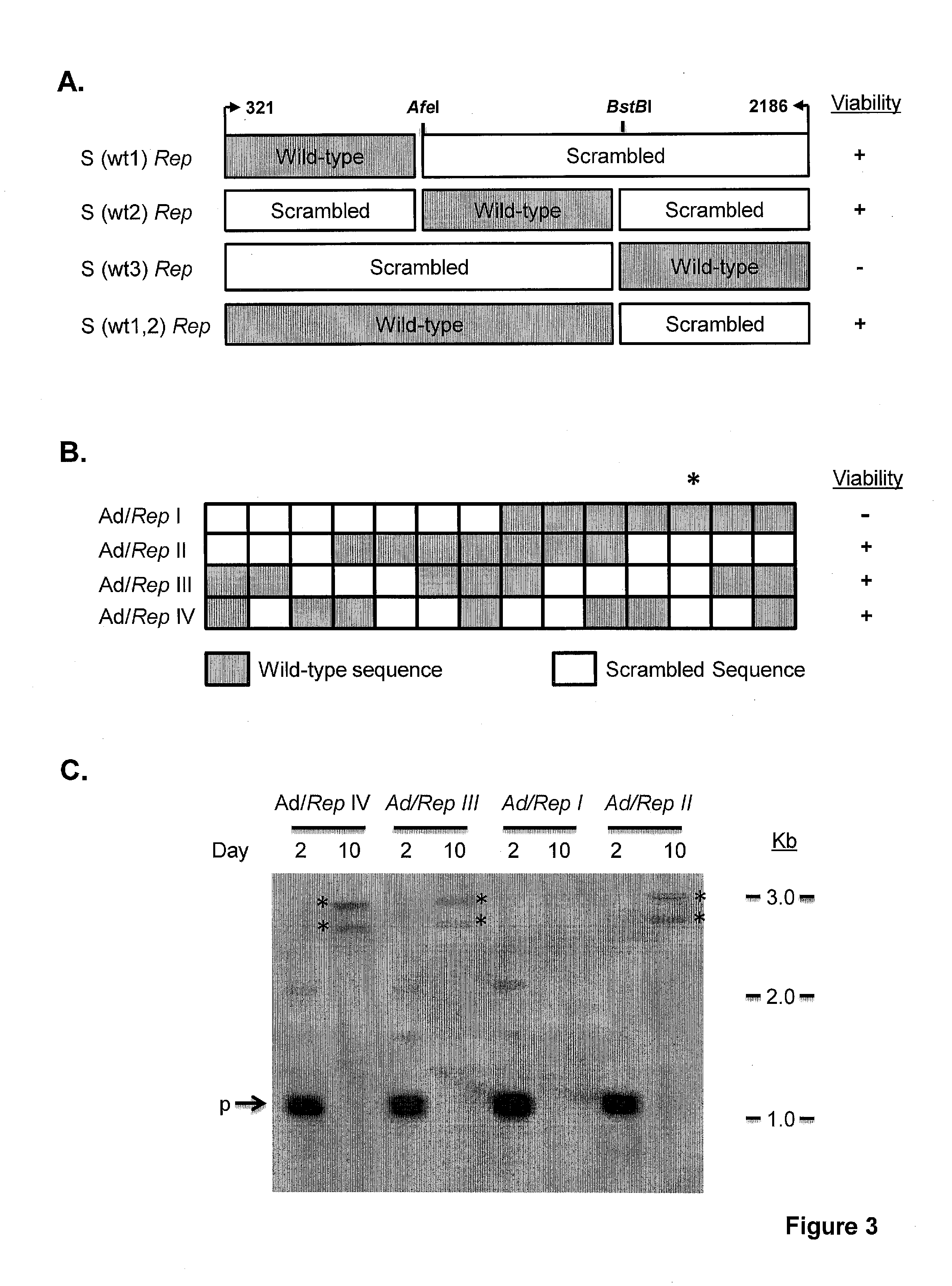
[0253]All de novo synthesized sequences were synthesized and cloned into pUC13 by GenScript USA. Scrambled Rep, Deoptimized Rep, were designed using a program previously described (13). The sequences were designed with unique restriction sites SbfI and SwaI flanking them as well as a unique R.E AfeI at bp 661 of the AAV2 genome to allow ease of manipulation. A naturally occurring R.E BstBI site that occurs at bp 1623 of the AAV2 genome was retained.
[0254]Rep78 Design I, II, III and IV was designed using algorithms described in the art (Coleman et al., Science 320:1784 (2008). S (wt3) Rep contains wild type Rep sequences from AAV2 bp 1623 to 2186. S (wt1,2) Rep contains scrambled Rep sequences from AAV2 bp 1623 to 2186. The constructs shown in FIG. 3 delimit the 135 bp interval from bp 1782 to bp 1916 of the AAV2 genome.
Plasmids and Cloning:
[0255]For detection of expression levels from wild-type, scrambled and Deoptimized Rep ORFs, ...
example 2
Modification of Rep ORF
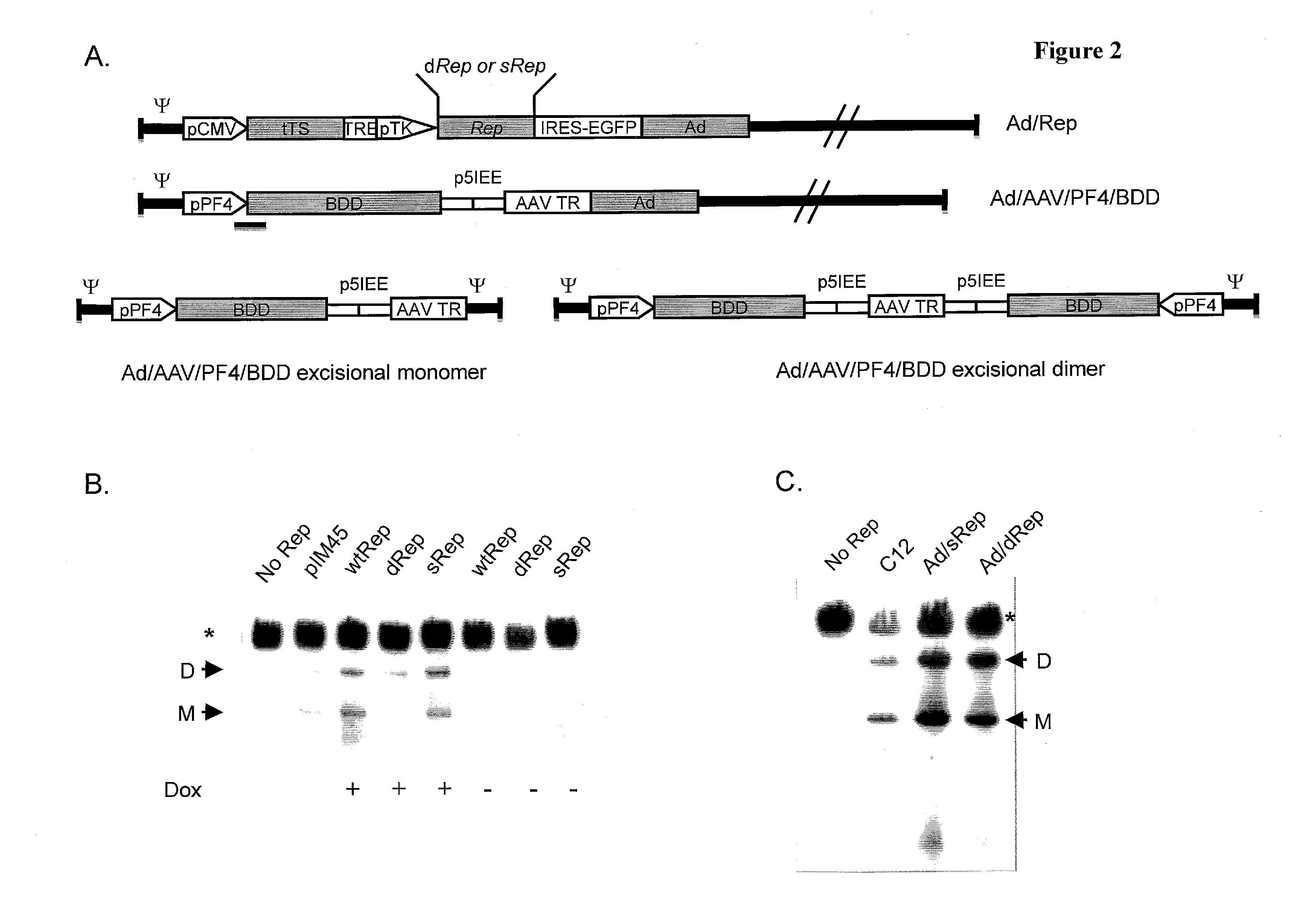
[0267]To construct a first generation Ad carrying Rep78, the inventors expressed Rep under a tightly regulated tetracycline inducible promoter within an ΔE1ΔE1 F5 / 35 Adenovirus. The fiber knob of this Ad5 was replaced with that of Ad35 to allow it to infect hematopoietic cells (3). The tetracycline inducible Rep78 expression cassette, has been previously used successfully for the construction of a helper dependent Adenovirus carrying Rep78 (9). Surprisingly, the inventors found that the same construct on an E1 deleted backbone was incapable of replication, showing no signs of viral growth in spite of multiple passages in HEK 293 packaging cells. The inventors hypothesized that the replicative functions provided by multiple helper virus genomes in trans to the helper dependent virus allowed replication, whereas a single genome carrying both Adenoviral genes and the Rep expression construct was unable to escape Rep's inhibitory effect.
[0268]To elucidate the rela...
example 3
Modification of Rep ORF Allows Replication of Adenovirus
[0271]The Scrambled and Deoptimized Rep constructs were cloned downstream of the tetracycline inducible promoter, in place of the wild-type Rep ORF, within the fiber modified first generation Adenovirus genome, generating infectious clones pAd / sRep78 and pAd / dRep78 (FIG. 2A). These viral constructs were linearized and transfected into HEK 293 packaging cells and passaged every 10 days onto fresh cells, until the development of CPE was observed. As mentioned earlier, no signs of viral replication could be observed with pAd / WTRep78 even with passaging up to 50 days. However, complete CPE was observed with both pAd / sRep78 and pAd / dRep78 within a total of 15 days from transfection. Production of both viruses could be scaled up with infectious virus yields comparable to each other and to Ad / AAVFVIII (Table 1), proving a clear role for the sequence of Rep in the inhibition of Adenoviral replication.
TABLE IViral titersVirusTiter† (PFU...
PUM
| Property | Measurement | Unit |
|---|---|---|
| genetic defects | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com