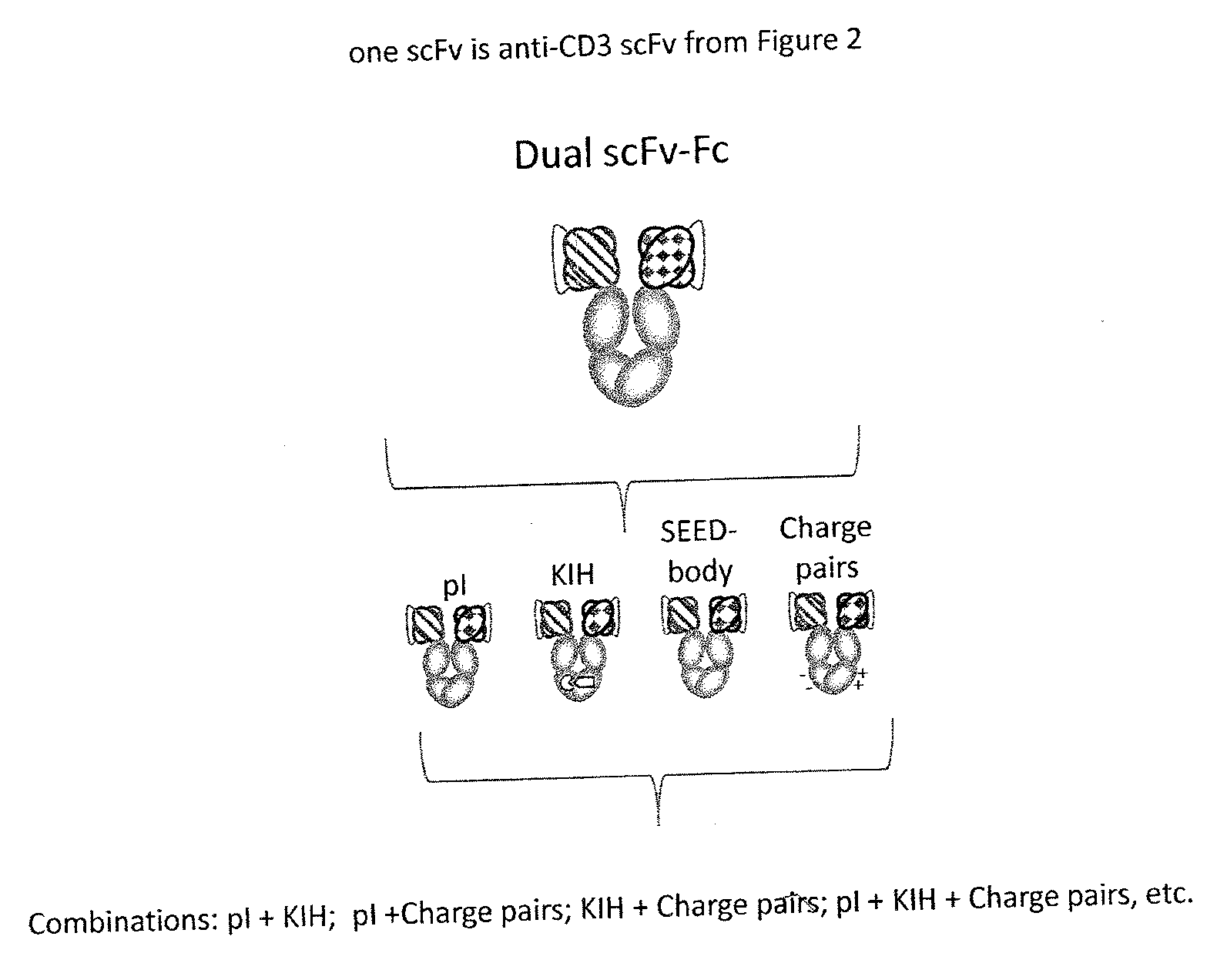
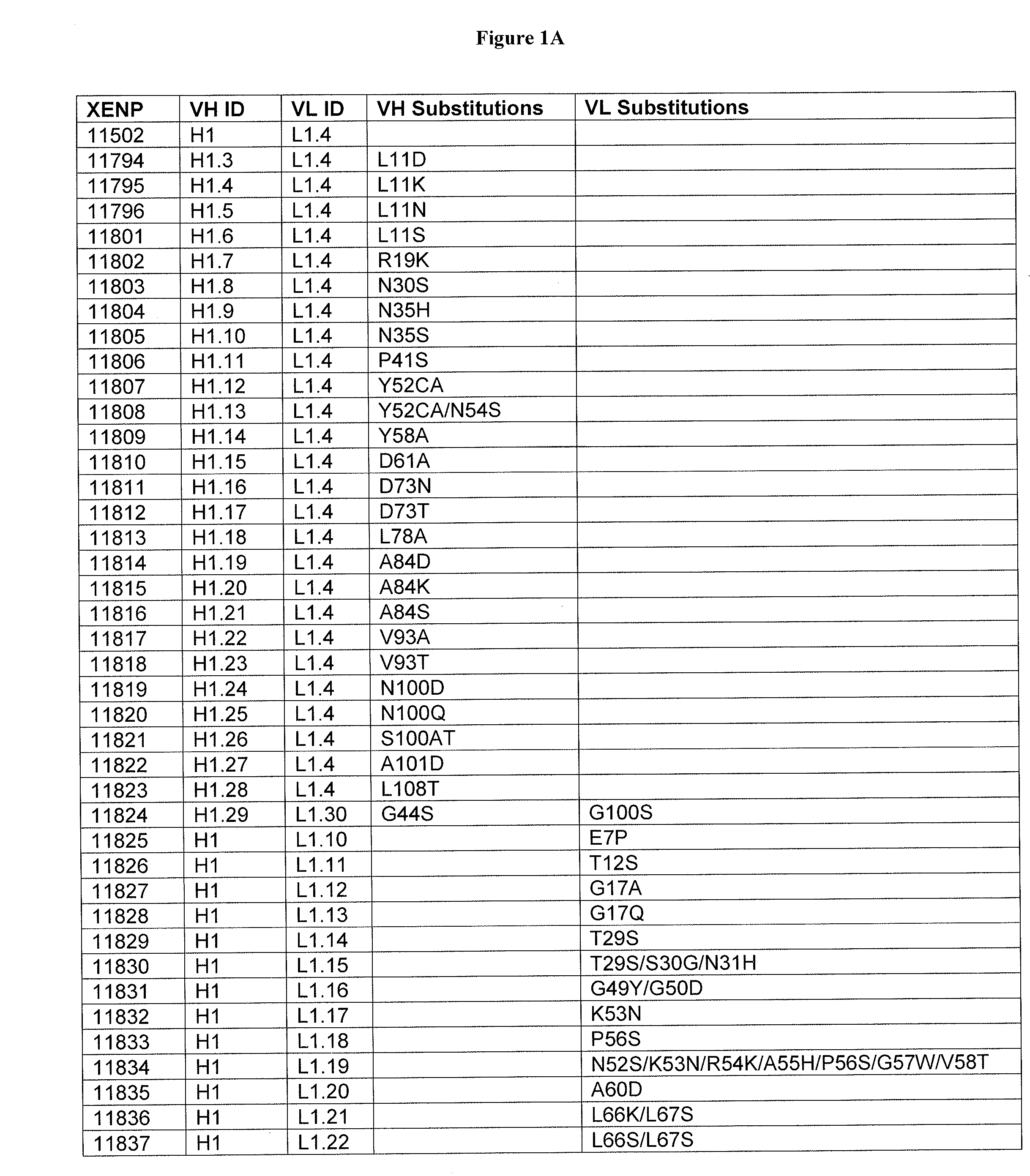
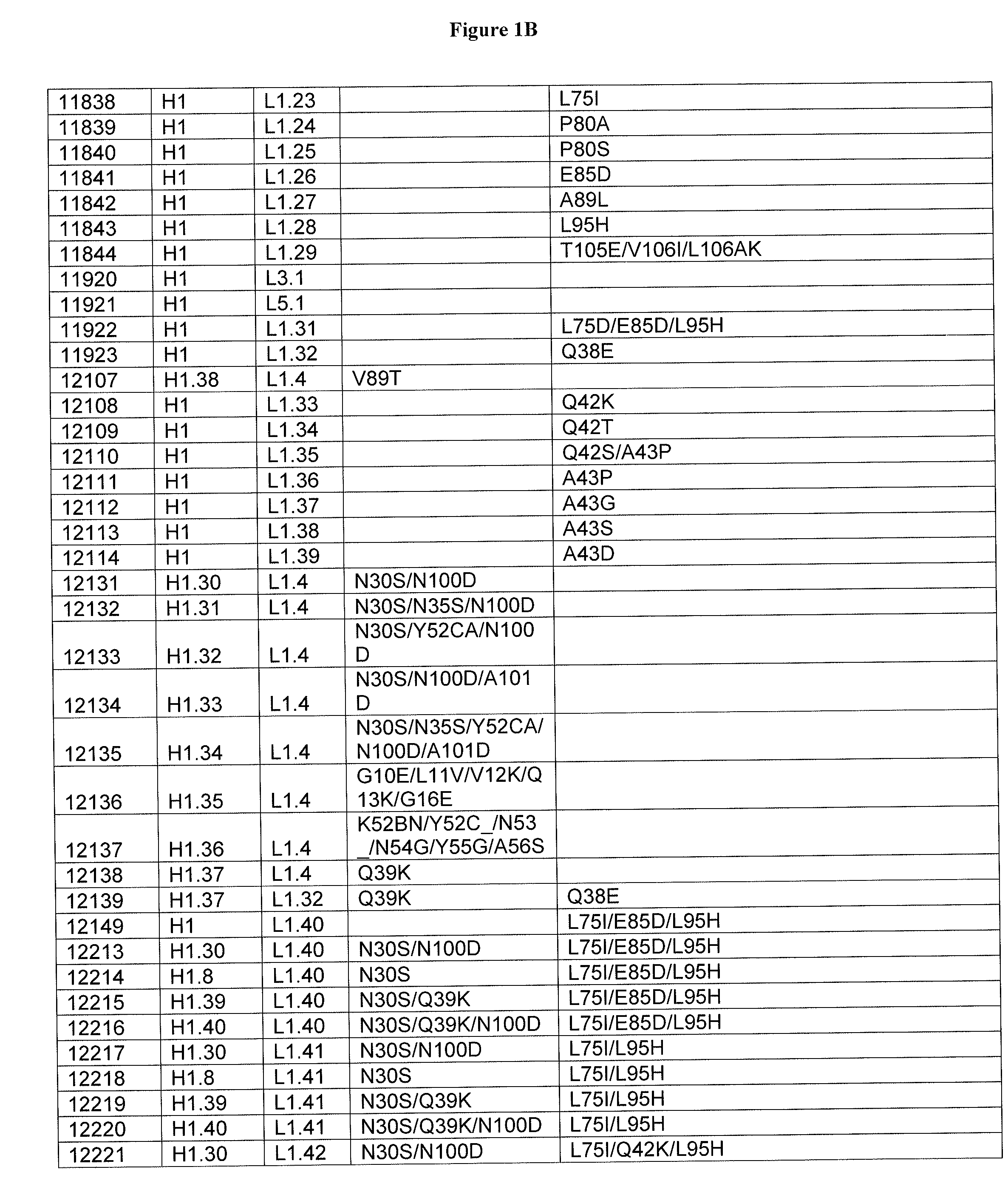
Optimized Anti-cd3 variable regions
a variable region and anticd3 technology, applied in the field of optimizing anticd3 variable region, can solve the problems of affecting the production and stability of antibody fragments, unable to have the constant region of the antibody with its associated functional properties, and the binding to the new antigen is always bivalen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
scFv Production
[0299]DNA encoding the anti-CD3 scFv with a C-terminal GlySer linker and His6-tag was generated by gene synthesis (Blue Heron Biotechnology, Bothell, Wash.) and was subcloned using standard molecular biology techniques into the expression vector pTTS. Substitutions were introduced using either site-directed mutagenesis (QuikChange, Stratagene, Cedar Creek, Tex.) or additional gene synthesis and subcloning using a BspEI restriction site placed in the (Gly4Ser)3 VH-VL linker. DNA was transfected into HEK293E cells for expression and resulting proteins were purified from the supernatant using nickel affinity (Qiagen, Valencia, Calif.) chromatography.
[0300]Determination of Tm
[0301]Differential scanning fluorimetry (DSF) experiments were performed using a Bio-Rad CFX Connect Real-Time PCR Detection System. Proteins were mixed with SYPRO Orange fluorescent dye and diluted to 0.2 mg / mL in PBS. The final concentration of SYPRO Orange was 10×. After an initial 10 minute incuba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com