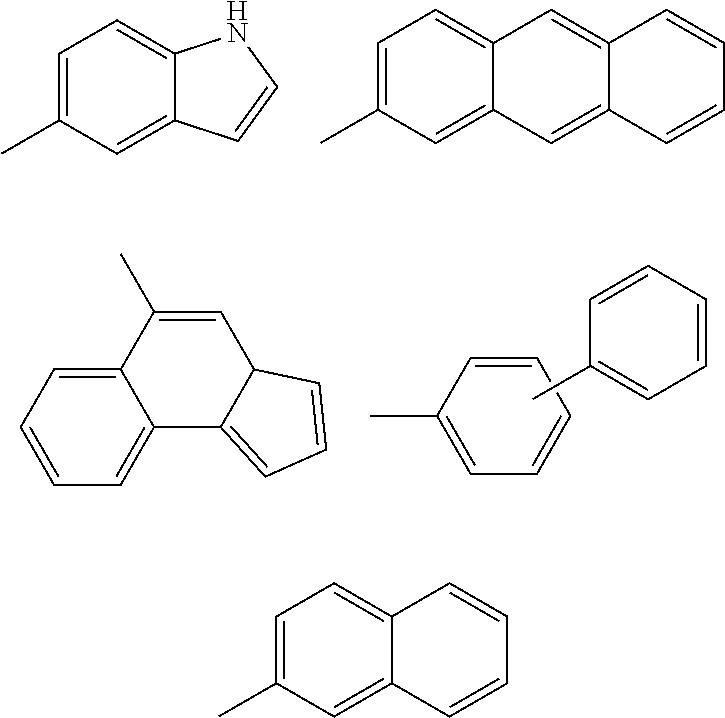
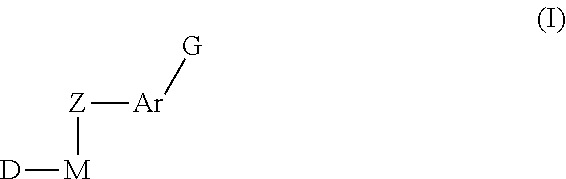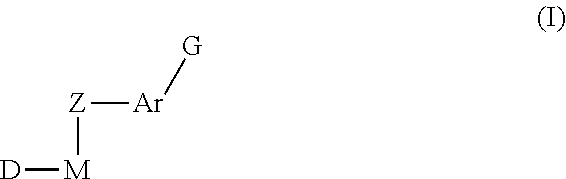Methods of treatment of cell proliferative and/or ophthalmic diseases, disorders and conditions using inhibitors of protein tyrosine kinase activity
a technology of protein tyrosine kinase and inhibitors, which is applied in the field of compound medicines, can solve the problems of limiting this approach, affecting the effect of vegf inhibitors as cancer therapeutics, and vision loss, and achieves the effects of inhibiting kinase activity, and inhibiting protein tyrosine kinase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 6
N-((6-(7-(4-(3-cyclopropylureido)-2-fluorophenoxy)thieno[3,2-b]pyridin-2-yl)pyridin-3-yl)methyl)-N-(2-methoxyethyl)formimidamide
[1029]A suspension of 1 (150 mg, 0.296 mmol) and ethylformimidate hydrochloride (130 mg, 1.18 mmol) in MeCNEtOH (10 mL / 5 mL) was heated to reflux overnight. More ethylformimidate hydrochloride (130 mg, 1.18 mmol), MeCN (20 mL) and EtOH (10 mL) were added, and the reaction mixture was heated to reflux overnight then cooled to RT. Finally the reaction mixture was concentrated and partitioned between AcOEt and water. The aqueous layer was concentrated (the desired compound is water-soluble at pH around 4-5). The residue was purified by Gilson (Phenomenex, Luna, 15μ, C18(2) 100A, 250×50.00 mm, 15 μm, 0.05% of formic acid in both MeOH / water: 20 / 80 to 95 / 5 over 60 min, flow=30 mL / min), to afford the title compound 7 (30 mg, 0.056 mmol, 23% yield) as a yellow-mustard solid. 1H NMR (400 MHz, DMSO-d6) δ (ppm): mixture of isomers and / or rotamers, 9.62-9.48 (m, 0.5H),...
example 7
1-cyclopropyl-3-(3-fluoro-4-(2-(5-(((2-methoxyethyl)(2,2,2-trifluoro ethyl)amino)-methyl)pyridin-2-yl)thieno[3,2-b]pyridin-7-yloxy)phenyl)urea
[1030]A solution of 1 (150 mg, 0.296 mmol), DIPEA (0.3 mL, 1.72 mmol) and 2-iodo-1,1,1-trifluoroethane (2 mL, 20.29 mmol) in DMSO (4 mL) was stirred at 110° C. overnight, then cooled to RT. The reaction mixture was partitioned between AcOEt and water. The organic layer was washed with a saturated aqueous solution of ammonium chloride, a saturated aqueous solution of sodium bicarbonate, dried over anhydrous magnesium sulfate, filtered and concentrated. The residue was purified three times by Biotage (SNAP 12 g cartridge; MeOH / DCM: 00 / 100 to 10 / 90 over 20 CV, then 10 / 90 over 5 CV) and then by Gilson (Phenomenex, Luna 15μ C18(2) 100A, 250×50.00 mm, 15 μm, 0.05% formic acid in both MeOH / water: 60 / 40 to 95 / 5 over 60 min, flow=30 mL / min), to afford the title compound 8 (2.6 mg, 0.004 mmol, 2% yield) as a colorless sticky film. 1H NMR (400 MHz, MeCN-...
example 8
1-cyclopropyl-3-(3-fluoro-4-(2-(5-((2-hydroxyethylamino)methyl)pyridin-2-yl)-thieno[3,2-b]pyridin-7-yloxy)phenyl)urea
[1031]To a solution of 1 (400 mg, 0.788 mmol) in anhydrous DCM under nitrogen was slowly added BBr3 in DCM (6.3 mL, 1.0 M) at −50° C. The reaction mixture was allowed to warm to RT over 5 h. The reaction mixture was then quenched by addition of methanol and concentrated. The residue was dissolved in a mixture of methanol / 1N HCl / DMSO and purified twice by Gilson (Phenomenex, Luna, 15μ, C18(2) 100A, 250×50.00 mm, 15 μm, 0.05% of formic acid in both MeOH / water: 20 / 80 to 95 / 5 over 60 min, flow=30 mL / min), then (Phenomenex, Luna, 15μ, C18(2) 100A, 250×50.00 mm, 15 μm, 0.05% of formic acid in both MeOH / water: 20 / 80 to 70 / 30 over 60 min, flow=30 mL / min), to afford the title compound 9 (53 mg, 0.107 mmol, 15% yield, formate salt) as an off-white solid. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.12 (s, 1H), 8.60 (d, J=1.4 Hz, 1H), 8.52 (d, J=5.5 Hz, 1H), 8.32 (s, 1H), 8.30-8.21 (m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com