Methods for treating visceral fat conditions
a technology for visceral fat and conditions, applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems the presence of visceral fat and its health consequences, so as to reduce the high-density lipoprotein (hdl) cholesterol level, increase and reduce the risk and/or severity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0039]A double-blind, placebo-controlled multi-center trial was conducted with 285 healthy, non-diabetic, obese subjects. The subjects were administered either bupropion 200 mg bid, placebo (P), naltrexone 48 mg qd (N1), or bupropion 400 mg with naltrexone 32 mg qd (BN2). 182 subjects completed 24 weeks of treatment. A subset of 60 subjects had dual energy X-ray absorptometry (DEXA) and multislice CT scans to measure body fat, lean tissue and visceral fat (American Diabetes Association Annual Meeting 2007).
[0040]The groups were matched at baseline. Markers of insulin resistance improved more with BN2 than expected from the weight loss alone. A robust effect on decreasing visceral fat was also evident.
TABLE 1Naltrexone &BupropionPlaceboNaltrexone 48 mgBupropion 400 mg32 / 400 mgWeight (%) −1.1 ± 0.6***−1.74 ± 0.9*** −3.14 ± 0.7***−7.1 ± 0.7Waist (cm)−1.0 ± 5.4**−3.8 ± 12.7 −2.9 ± 6.0−5.4 ± 7.6Fasting Glucose1.9 ± 1.3*3.4 ± 1.7* 3.5 ± 1.5*−2.0 ± 1.5(mg / dL)Insulin 0.9 ± 0.9** 1.7 ± 1.3**...
example 2
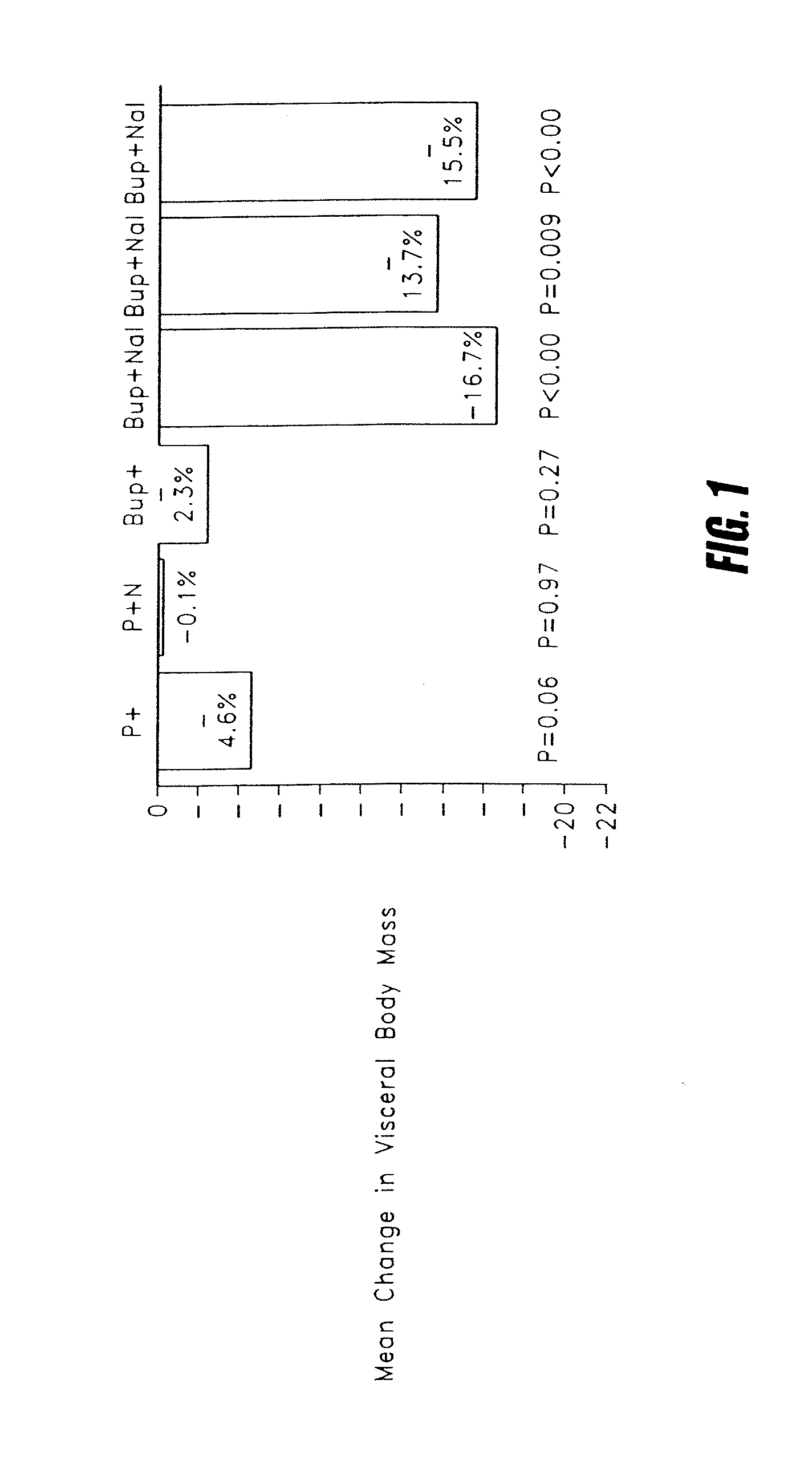
[0041]Subjects (n=117) received one of six treatments: two placebos (P+P), placebo and naltrexone (P+Nal), bupropion and placebo (Bup+P), bupropion and naltrexone 48 mg (Bup+Nal 48), bupropion and naltrexone 32 mg (Bup+Nal 32), or bupropion and naltrexone 16 mg (Bup+Nal 16).
[0042]Subjects had a DEXA body scan to measure total body fat, lean tissue and bone mineral content at baseline and at 6 months. Subjects also had a multi-slice CT scan to determine visceral fat volume at the same time points. The mass of selective visceral loss can be calculated based on total fat and the volume of visceral fat.
[0043]The average change in visceral body mass is shown in FIG. 1 for the four treatments. Patients receiving Bup+Nal 32 experienced a dose-related loss in visceral body mass. Statistically significant improvements were also observed in several important metabolic parameters including: plasma glucose, serum insulin and plasma triglycerides.
TABLE 2Baseline Weight, BMI and Weight Circumfere...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com