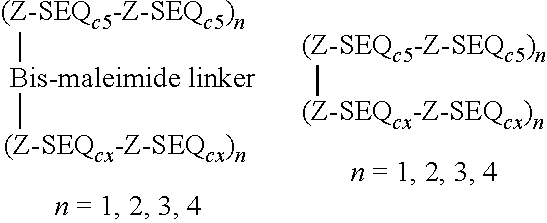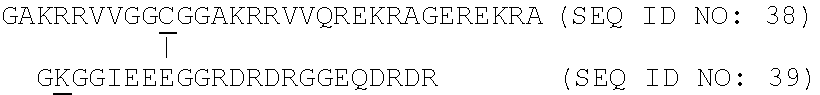Vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
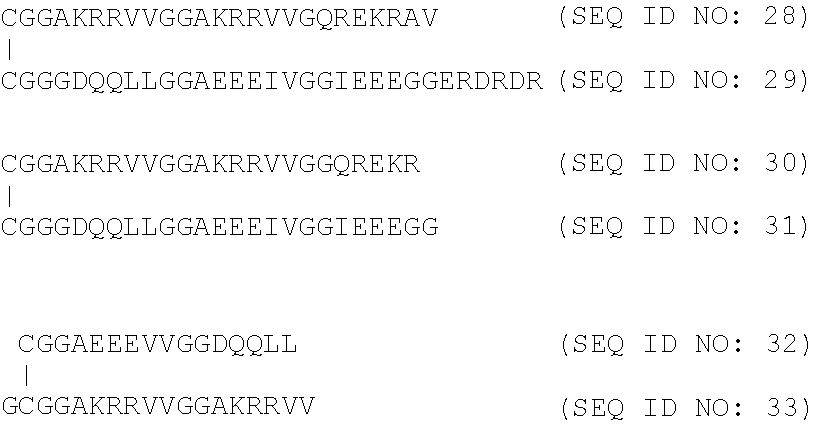
[0226]Peptides to be used according to the present invention that elicit Cell-mediated immunity (CMI) or peptides that stimulate the humoral immunity in a subject were synthesized using conventional techniques for linear sequences as described in international patent applications WO0052040, WO 2012 / 092934 or WO 2012 / 072088.
[0227]Specifically compounds to stimulate the humoral immunity in a subject by inducing antibodies that stabilise association of the C5 domain of HIV gp120 with the transmembrane domain of gp41 and / or with the constant C2 domain of gp120 were prepared as described in WO2011 / 000962.
[0228]Also compounds to stimulate the humoral immunity in a subject by stabilising the association of the C5 domain of HIV gp120 with the transmembrane domain of gp41 and / or with the constant C2 domain of gp120 were prepared as described in WO2011 / 000962.
[0229]Immunomodulatory compound and a reservoir purging agent, such as a histone deacetylase (HDAC) inhibitor used according to the pre...
example 2
[0439]A vaccine comprising the peptides of the SEQ ID NO:49, 52, 57 and 64 was prepared. The freeze-dried peptides are dissolved in sterile water at a final concentration of 4 mg / ml. The final salt concentration was 0.9%. A preparation of a granulocyte-macrophage-colony stimulating factor (GM-CSF) was also prepared, according to the manufacturers directions for use, to a final concentration of 0.3 mg / ml. The two solutions are administered intracutaneously. A typical injection dose is 100 μl.
example 3
[0440]An antigen solution or suspension is mixed with equal parts of Freund's adjuvant of Behring, complete or incomplete, and is then finely emulsified by being drawn up into, and vigorously pressed out of, an injection syringe, or with a homogenator. The emulsion should remain stable for at least 30 minutes. The antigen-adjuvant emulsions is best injected subcutaneously as a depot.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com