Disease progression parameters and uses thereof for evaluating multiple sclerosis
a disease progression and disease technology, applied in the field of disease progression parameters, can solve the problems of reduced ability to remyelinate, loss of trophic factors supporting neurons and axons, and damage to nerve fibers, so as to improve the response to worsening, improve the scoring accuracy and consistency, and increase sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
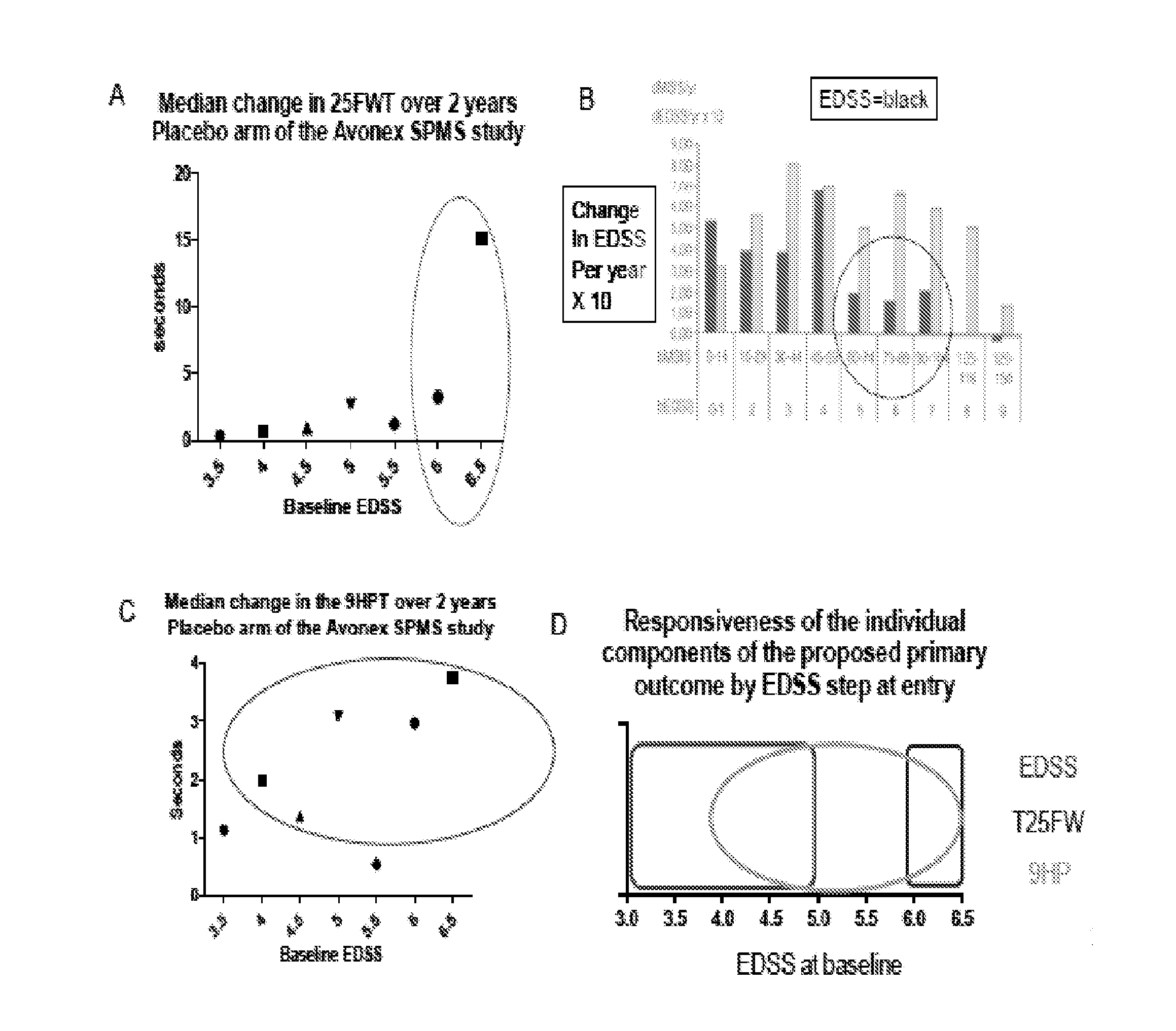
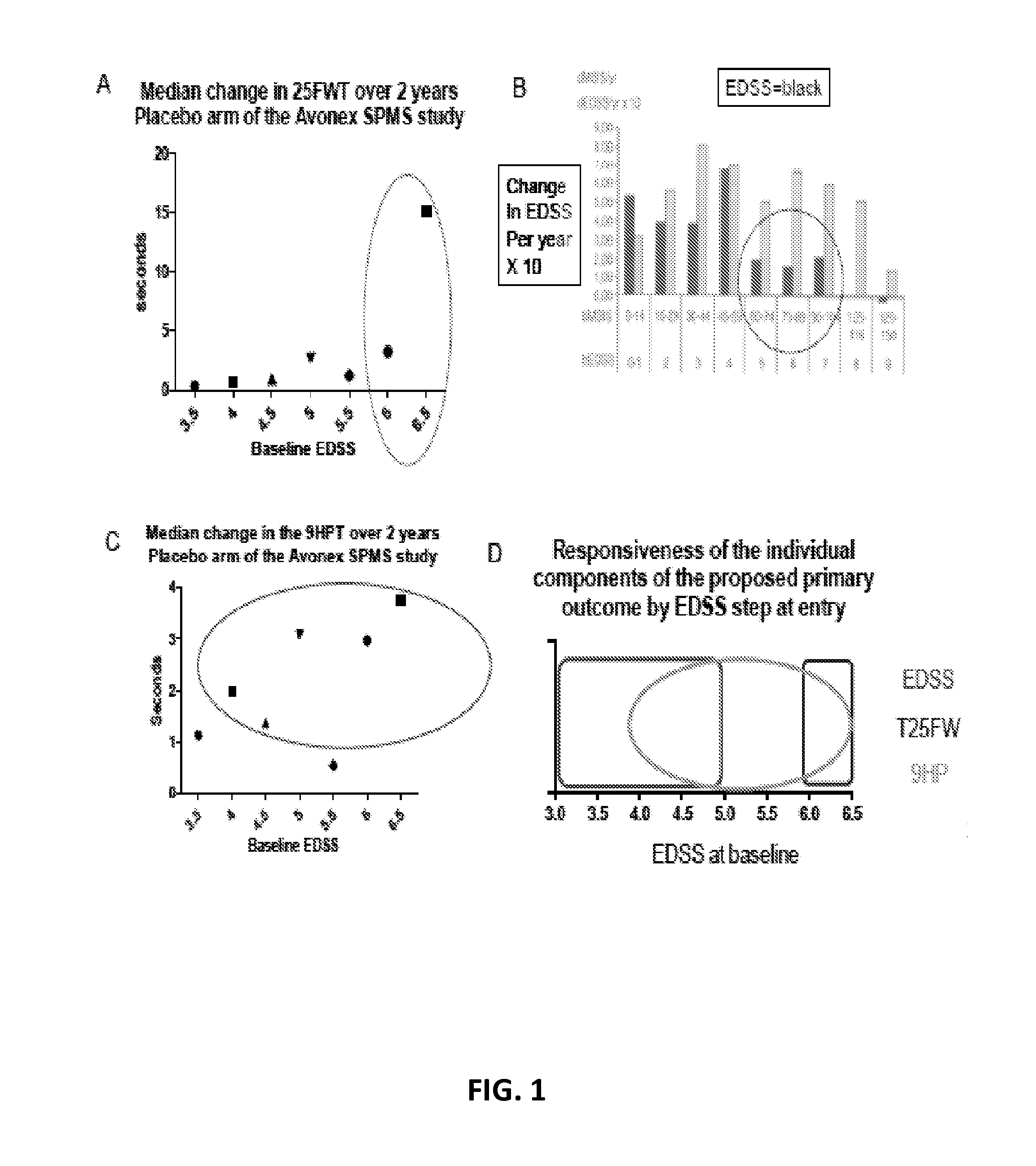
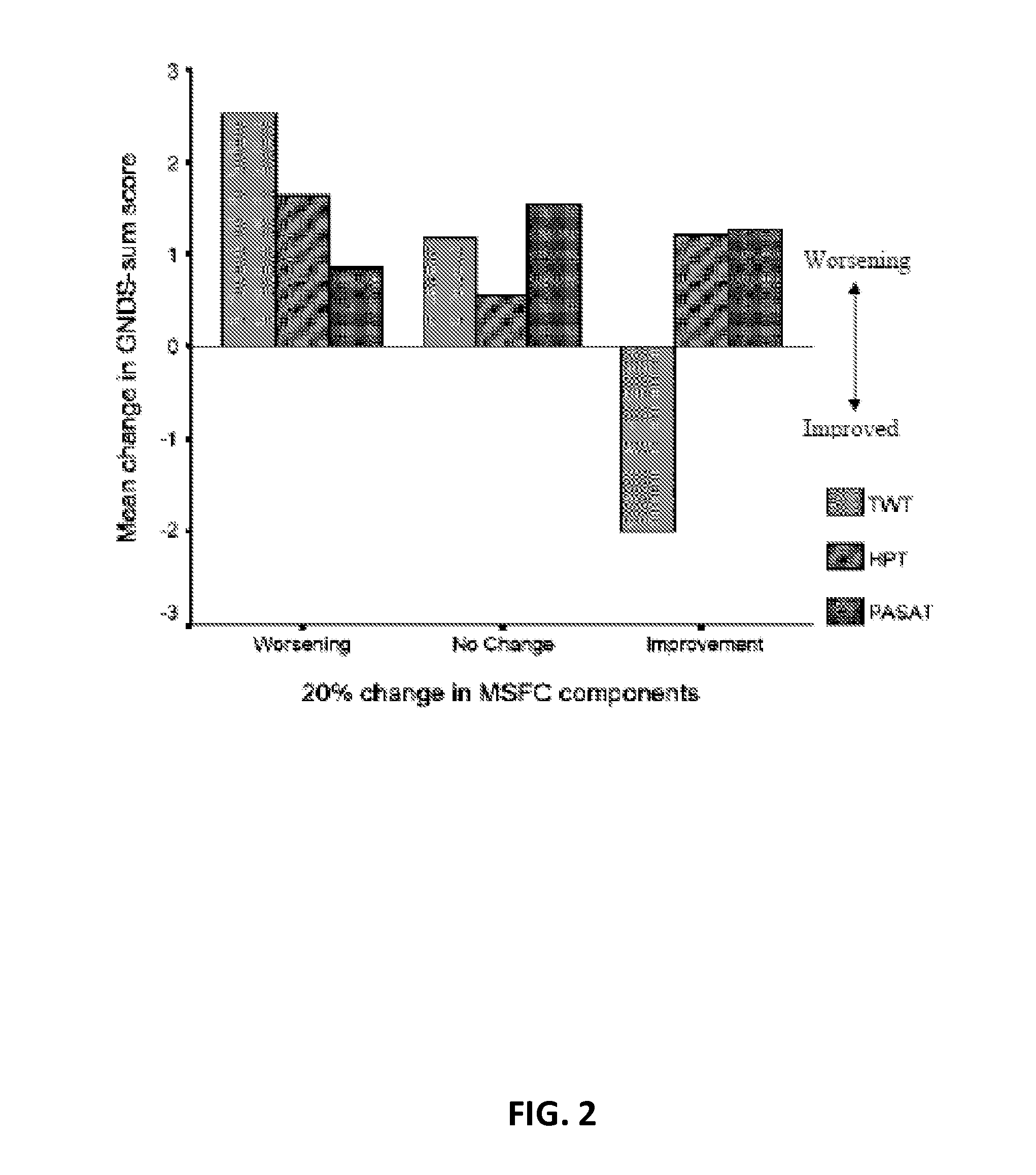
[0197]Current methodologies for evaluating MS patients, e.g., the EDSS, have been useful in RRMS clinical research because they can capture changes across combinations of neurological systems in the MS patient population. However, these methodologies are less reliable when used to evaluate progressive forms of MS, e.g., SPMS, PPMS and PRMS (Cohen et al., (2002) Mult Scler 8(2): 142-154). For example, most of the patients enter SPMS clinical trials with baseline EDSS scores of 3.5 or higher, at which point progression is determined almost exclusively by large, threshold-based worsening of ambulation. Furthermore, over 50% of subjects enter SPMS clinical trials at EDSS steps of 6 or 6.5 where the EDSS is least responsive (FIG. 1B). As a result, the EDSS alone is inadequate to examine the therapeutic efficacy of drugs for progressive forms of MS. These limitations of the EDSS have been highlighted by several investigators over the last 20 years ((Ebers et al., (2008) Neurology 71: 624-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com