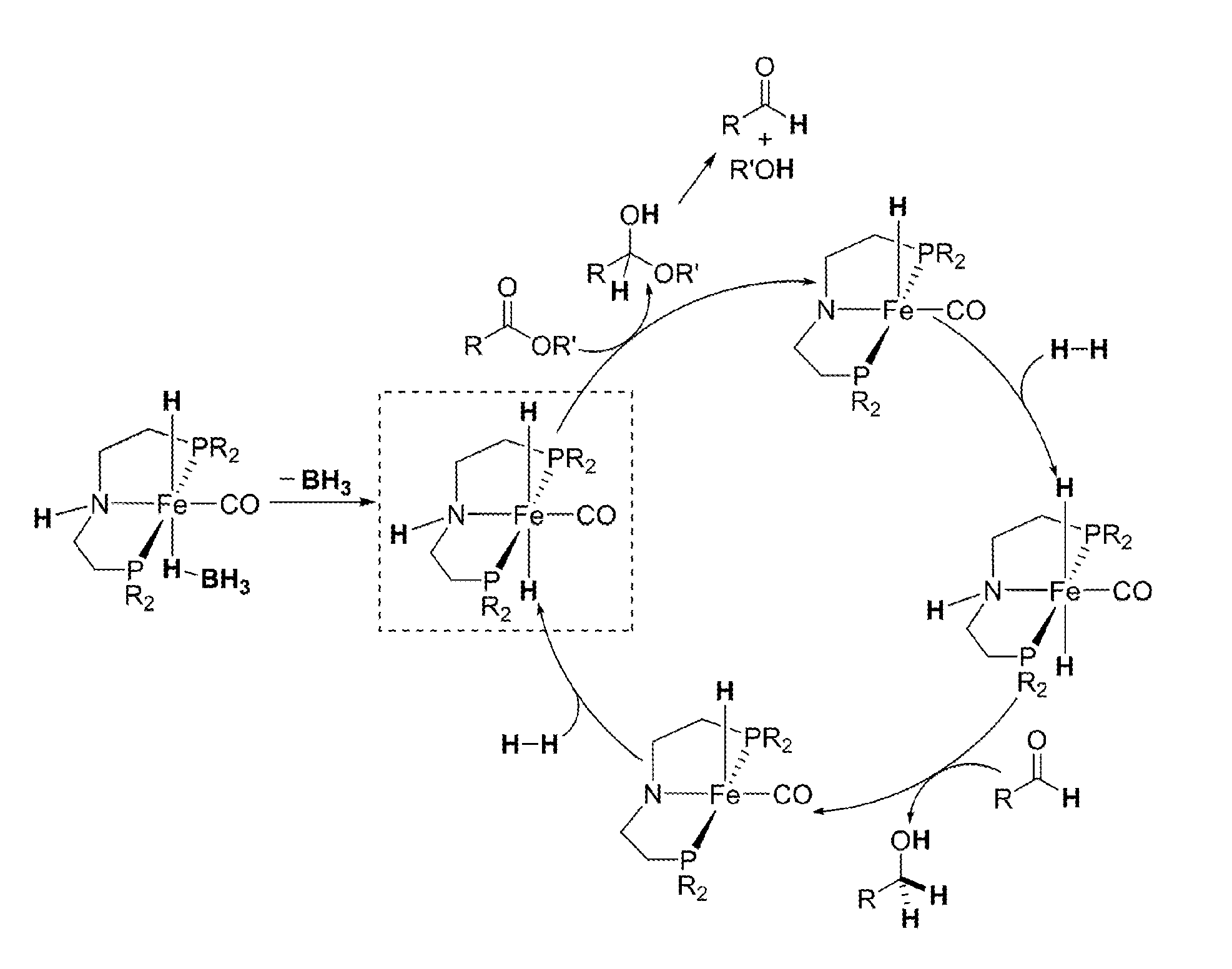
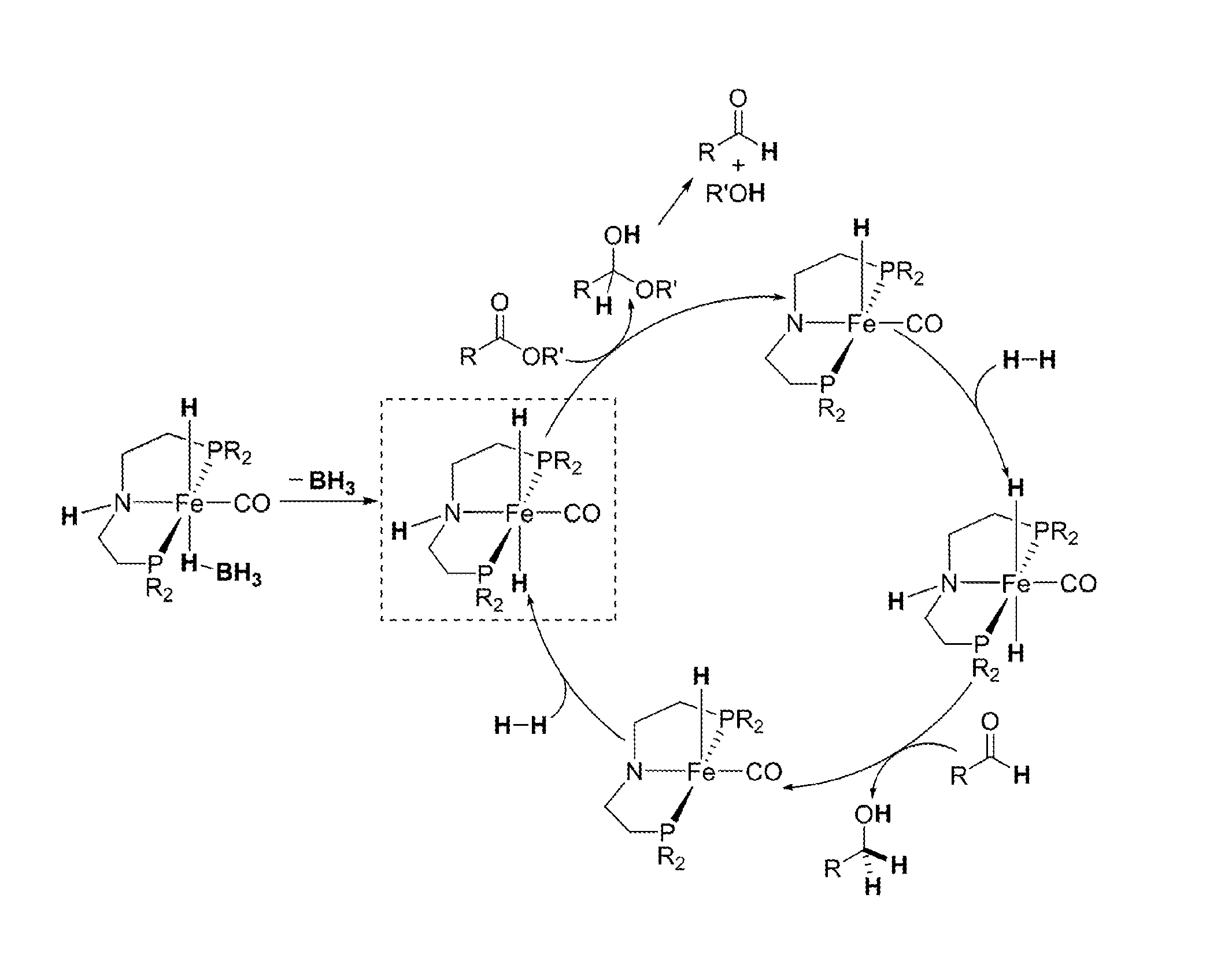
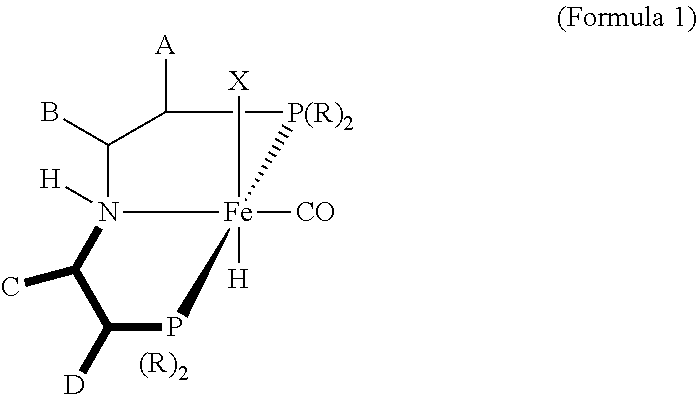
Homogeneous Hydrogenation of Esters Employing a Complex of Iron as Catalyst
a technology of complexes and iron, applied in the preparation of oxygen-containing compounds, organic compounds/hydrides/coordination complexes, physical/chemical process catalysts, etc., can solve the problems of high energy and capital expenditure of processes, and achieve the effect of reducing or minimizing the generation of harmful wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Catalyst Synthesis
Example 1A
Synthesis of [iPrPN(H)P]Fe(CO)Br2 (Formula 6)
[0020]In a glovebox, a 100 mL oven-dried Schlenk flask equipped with a stir bar was charged with anhydrous FeBr2 (510 mg, 2.36 mmol) and 30 mL of THF, which resulted in an orange solution. A THF solution of (iPr2PCH2CH2)NH (10 wt %, 9.0 mL, 2.60 mmol) was added and, upon mixing with the FeBr2 solution for a few minutes, a thick white precipitate formed. The flask was connected to a Schlenk line, and the argon inside the flask was replaced with CO by performing a freeze-pump-thaw cycle. When mixed with CO and warmed to room temperature, the white precipitate quickly dissolved to yield a deep blue solution. The solution was stirred under 15 psig of CO for 1 h followed by evaporation to dryness under vacuum. The resulting blue residue was washed with pentane (15 mL×3) and dried under vacuum to give the titled compound as a blue powder (1.20 g, 93% yield). The 1H NMR spectra of this complex showed broad resonances,...
example 1b
Synthesis of [iPrPN(H)P]Fe(H)(CO)(BH4) (Formula 2)
[0021]Under an argon atmosphere, a 100 mL oven-dried Schlenk flask equipped with a stir bar was charged with Formula 6 (400 mg, 0.73 mmol) and NaBH4 (138 mg, 3.65 mmol). Adding 50 mL of dry and degassed ethanol to this mixture at 0° C. at first resulted in a green solution, which changed its color to yellow within a few minutes. The resulting mixture was gradually warmed to room temperature and then stirred for additional 16 h. Removal of the volatiles under vacuum afforded a yellow solid, which was treated with 80 mL of toluene and then filtered through a pad of Celite to give a yellow solution. Evaporating the solvent under vacuum yielded the desired compound as a bright yellow powder (250 mg, 85% yield). This compound can be exposed to air briefly without significant decomposition.
[0022][iPrPN(H)P]Fe(D)(CO)(BD4) (Formula 2-d5) were synthesized similarly from Formula 6 and NaBD4. 1H NMR (400 MHz, C6D6, δ): −19.52 (t, JP-H=50.4 Hz, ...
example 1c
Synthesis of [iPrPN(H)P]Fe(H)(CO)(Br) (Formula 7)
[0023]Under an argon atmosphere, a 100 mL oven-dried Schlenk flask equipped with a stir bar was charged with Formula 6 (100 mg, 0.182 mmol) and NaBH4 (7.0 mg, 0.185 mmol). Adding 15 mL of dry and degassed ethanol to this mixture at 0° C. at first resulted in a green solution, which changed its color to orange within a few minutes. The resulting mixture was gradually warmed to room temperature and then stirred for additional 16 h. Removal of the volatiles under vacuum afforded an orange solid, which was treated with 40 mL of toluene and then filtered through a pad of Celite to give an orange solution. After the solution was concentrated to ˜3 mL under vacuum, it was carefully layered with ˜10 mL of pentane and placed in a refrigerator (0° C.). Orange crystals of the desired compound formed within a day. Decantation of the top layer using a cannula followed by solvent evaporation afforded the titled compound (60 mg, 70% yield). This com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com