Thermally expandable resin composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
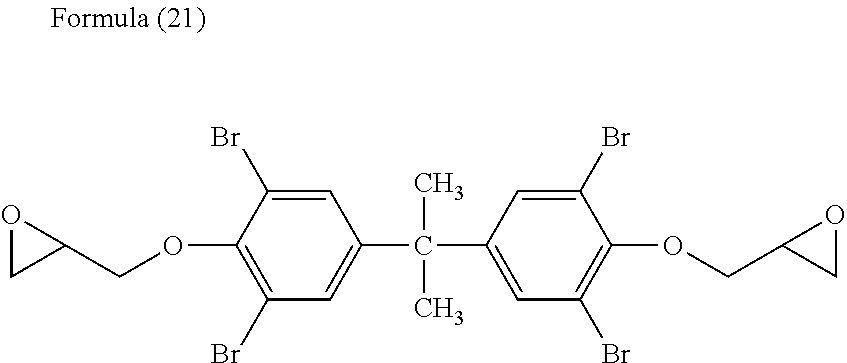
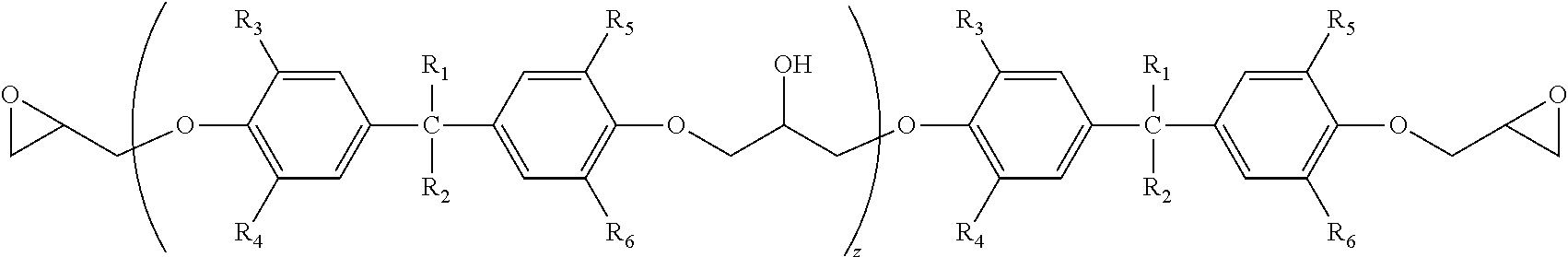
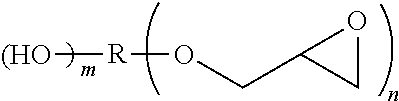
[0163][(Z) A Combination of Said Brominated Epoxy Epoxy Compound and at Least One of a Bisphenol-Type Epoxy Compound and an Aliphatic Epoxy Compound]
[0164](X is a repeating unit ranging from 1 to 100.)
[0165]10.9 parts by weight of tetrabromobisphenol-A-diglycidyl-ether (available from Nippon Steel Chemical Co., Ltd., trade name: YDB-400, an epoxy equivalent: 403.1 g / eq. Hereinafter referred to as “A-10”.) as the (Z-1),
[0166]45.4 parts by weight of bisphenol-F-diglycidyl-ether (available from Mitsubishi Chemical Corporation, trade name: E807, an epoxy equivalent: 168 g / eq. Hereinafter referred to as “A-1”.) as the (Z-3),
[0167]43.7 parts by weight of an amino compound which is prepared by mixing 3-lauryloxypropyl-1-amine with hexamethylenediamine derivative (available from Mitsubishi Chemical Corporation, trade name: FL052) at a weight ratio of 6:4 (an amine equivalent: 167.3 g / eq., on the basis of the active hydrogen. Hereinafter referred to as “B-1”.), as an epoxy curing agent, 90 p...
example 2
[0186]In the case of Example 1, 11.2 parts by weight of “A-10” are used, 39.3 parts by weight of “A-1” are used. Also, in addition to “A-10” and “A-1”, the (Z-6) is used.
[0187](R7 is an alkylene group having 1 to 20 carbon atoms, alkenylene group having 1 to 20 carbon atoms or arylene group having 1 to 20 carbon atoms.)
[0188]As the (Z-6), 4.7 parts by weight of dimer-modified epoxy (an epoxy equivalent: 422 g / eq. Hereinafter referred to as “A-3”.) are used.
[0189]Also, 43.9 parts by weight of “B-1” are used. The formulation used in Example 2 and the results are shown in Table 1.
example 3
[0190]In the case of Example 1, 31.6 parts by weight of “A-10” are used; the (Z-5) is used instead of “A-1”.
[0191]As the (Z-5), 31.5 parts by weight of hexamethylene diglycidyl ether (an epoxy equivalent: 157 g / eq. Hereinafter referred to as “A-2”.)
[0192]Also, completely the same experiment as in Example 1 is carried out, except that 36.9 parts by weight of “B-1” are used.
[0193]The formulation used in Example 3 and the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com