Maintenance medium for primate pluripotent stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
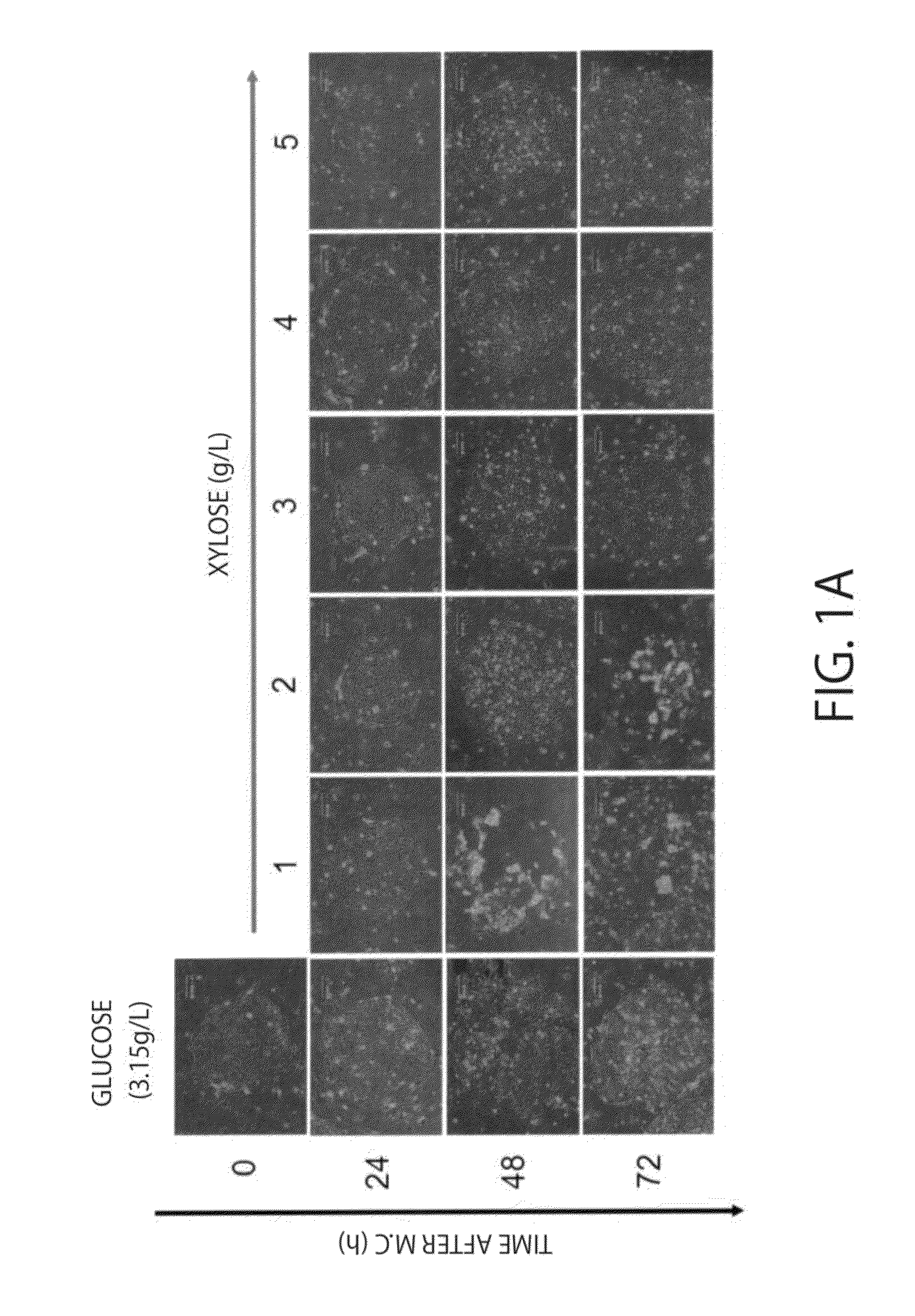
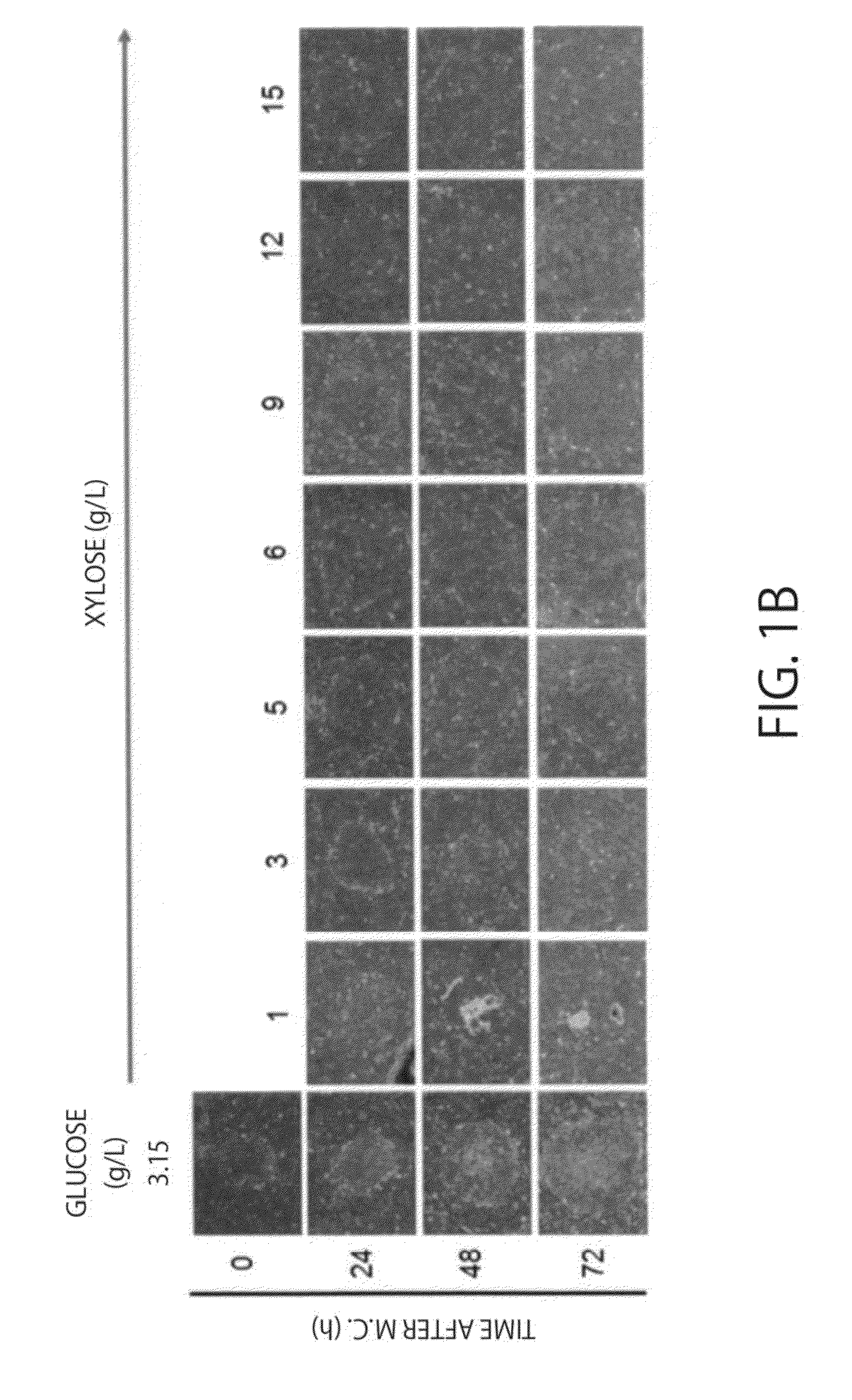
Culture of Human iPS Cells (253G1 Lines and 201B7 Lines) in a Xylose Medium
(1) Preparation of Medium
[0063]A medium for human iPS cells (induced pluripotent stem cells) was prepared each so that the composition was as follows.
Control Medium (Glucose Medium for iPS Cells):
[0064]DMEM / F12 (Gibco BRL, Rockville, Md.), 20 volume % KSR (Invitrogen), 2 mM L-glutamine (Gibco), 1×MEM non-essential amino acid solution (Wako), 100 μM β-mercaptoethanol (Sigma, St. Louis, Mo.), 50 U / mL penicillin and 50 μg / mL streptomycin (Gibco BRL), and 4 ng / mL basic FGF (Invitrogen).
Medium According to Present Invention (Xylose Medium for iPS Cells):
[0065]Modified DMEM / F12 containing D-xylose at various concentrations in place of glucose contained in a normal DMEM / F12 (M-DMEM, available from Nissui Pharmaceutical Co., Ltd.), 20 volume % KSR (Invitrogen) or 20 volume % dialysed KSR (available from Nissui Pharmaceutical Co., Ltd.) in which sugars (saccharides) are removed by a dialysis membrane (dialysis membran...
example 2
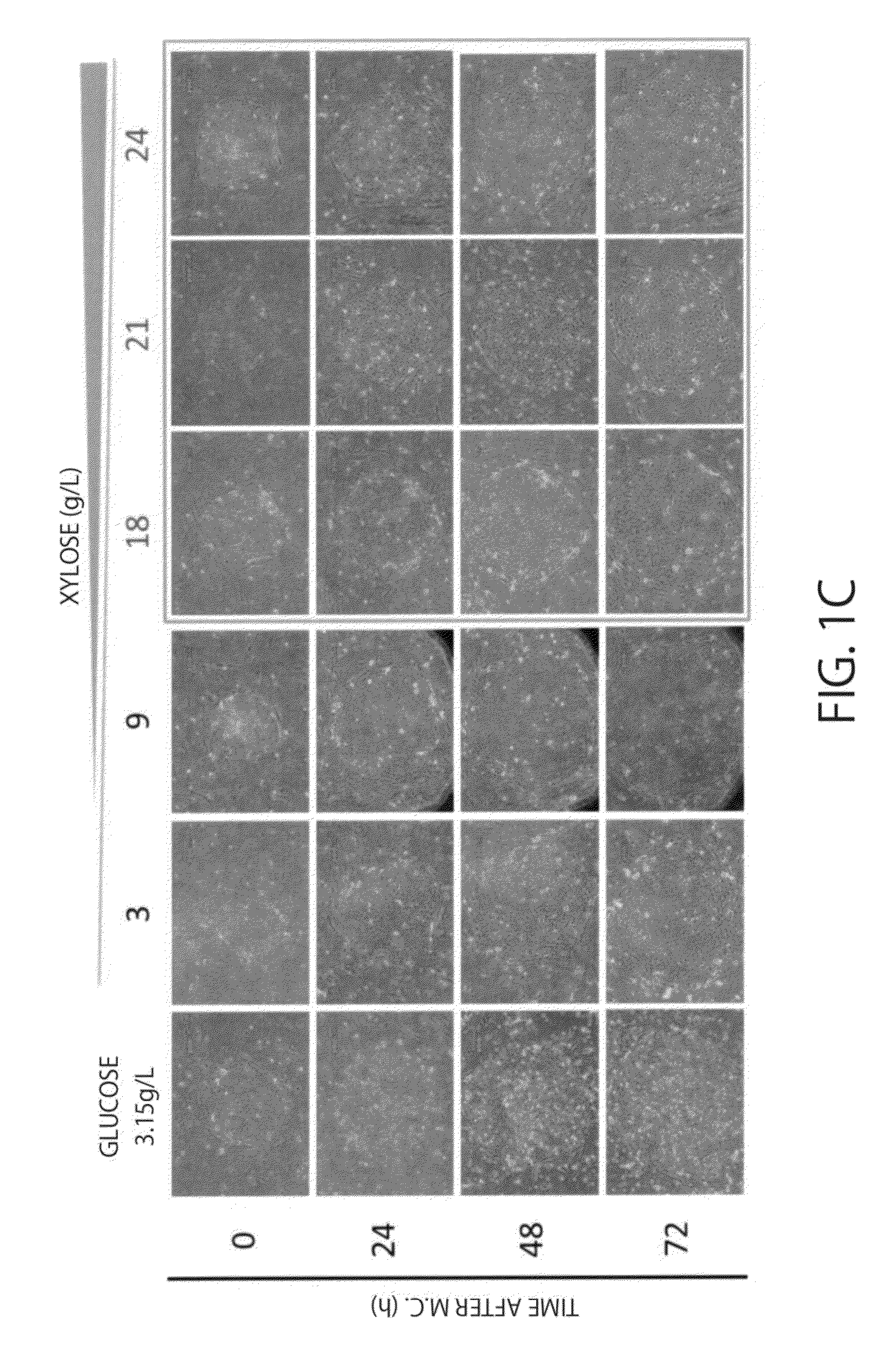
Cell Proliferation Control of Human iPS Cells
[0086](Disease iPS Cells Derived from Ehlers-Danlos Syndrome Patients (P-iPS Cells))
(1) Preparation of Medium
[0087]A medium for human iPS cells (induced pluripotent stem cells) was prepared each so that the composition was as follows.
Control Medium (Glucose Medium for iPS Cells):
[0088]DMEM / F12 (Gibco BRL, Rockville, Md.), 20 volume % KSR (Invitrogen), 2 mM L-glutamine (Gibco), 1×MEM non-essential amino acid solution (Wako), 100 μM β-mercaptoethanol (Sigma, St. Louis, Mo.), 50 U / mL penicillin and 50 μg / mL streptomycin (Gibco BRL), and 4 ng / mL basic FGF (Invitrogen).
Medium According to Present Invention (Xylose Medium for iPS Cells):
[0089]Modified DMEM / F12 in which glucose contained in a normal DMEM / F12 is changed with D-xylose (M-DMEM, available from Nissui Pharmaceutical Co., Ltd.) (D-xylose concentration: 3.15 g / L), 20 volume % dialysed KSR (available from Nissui Pharmaceutical Co., Ltd.) in which sugars are removed by a dialysis membran...
example 3
Metabolites of Human iPS Cells in Xylose Medium
(1) Saccharide Consumption in Each Medium
[0097]iPS cells (253G1 lines) were cultured for 24 hours with a xylose medium for iPS cells or a glucose medium for iPS cells, the consumption of each saccharide (sugar) contained in the supernatant after culture was calculated. Measurement of each saccharide was performed with HPLC (manufactured by Shimadzu Corporation). When the amount at 0 hours after culture was defined as 100.0, the sugar amount was 79.0 for a glucose medium for iPS cells and 88.2 for a xylose medium for iPS cells, and when converted into sugar consumption (%), it was 21.1% and 11.8%, respectively.
(2) Confirmation of Metabolites
[0098]For metabolites in cell samples when iPS cells (253G1 lines) were cultured in a xylose medium for iPS cells, metabolome analysis were performed. Specifically, cell samples and culture supernatants were deproteinized by ultrafiltration, and the relative area of detected substances among metabolit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com