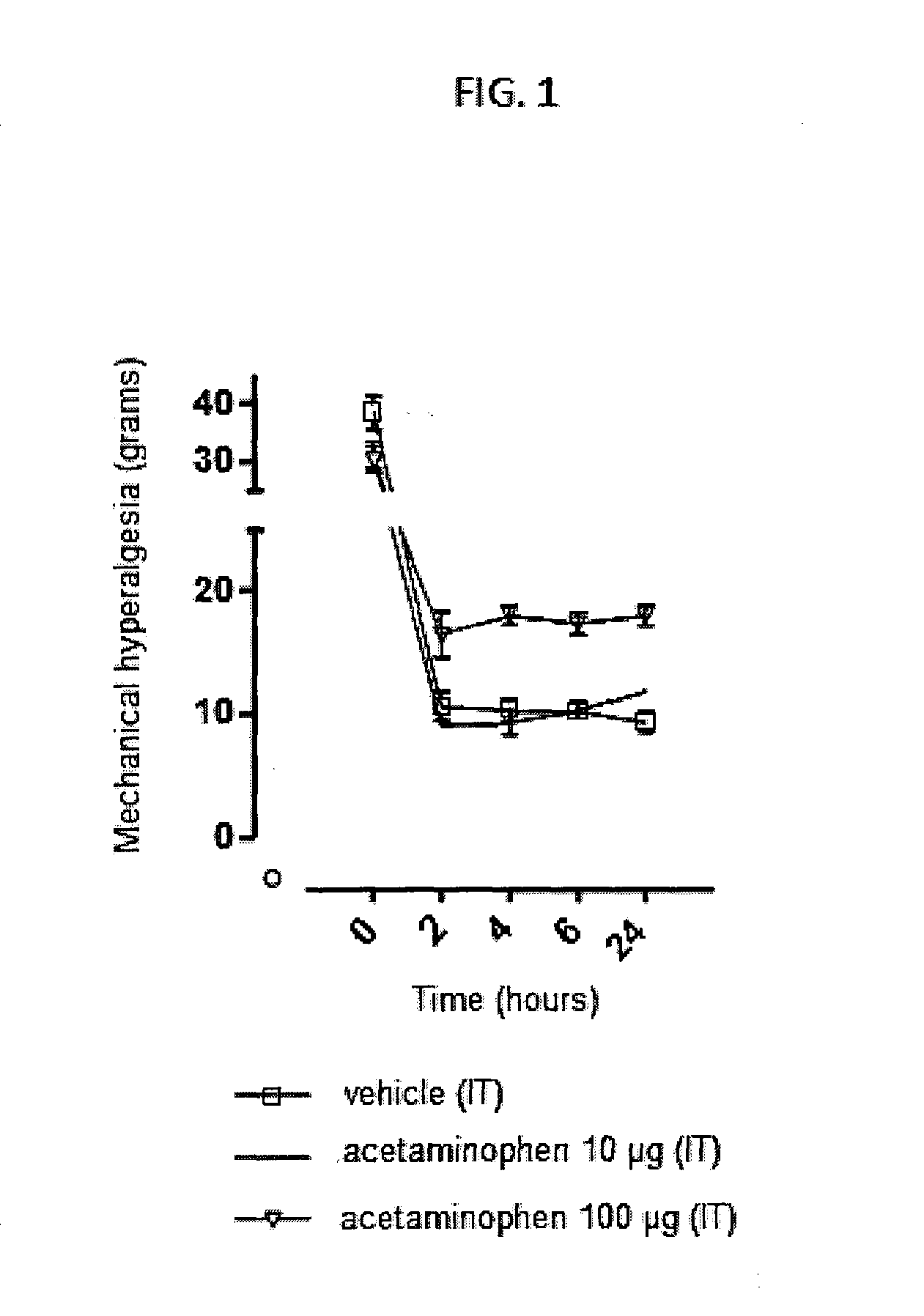
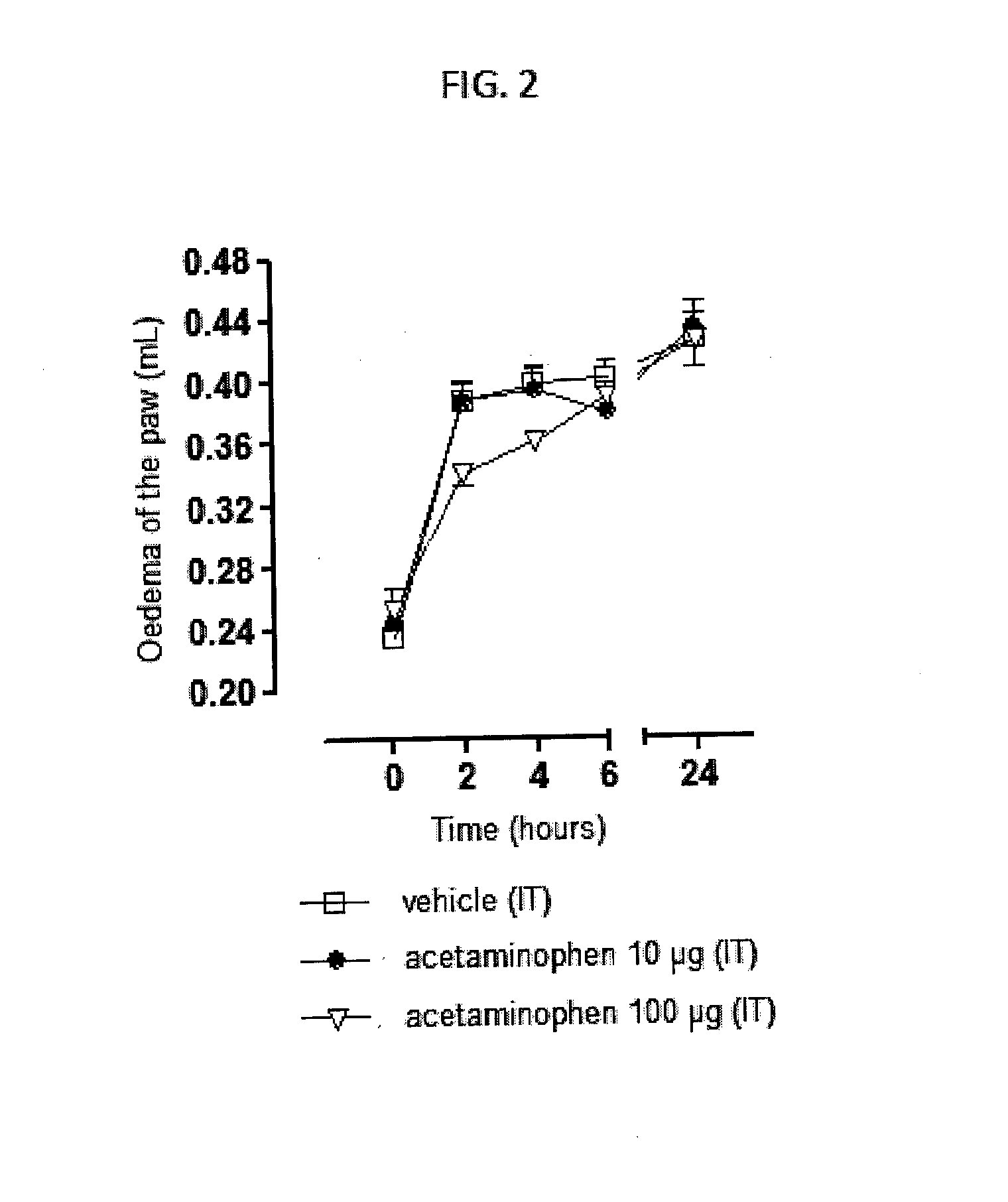
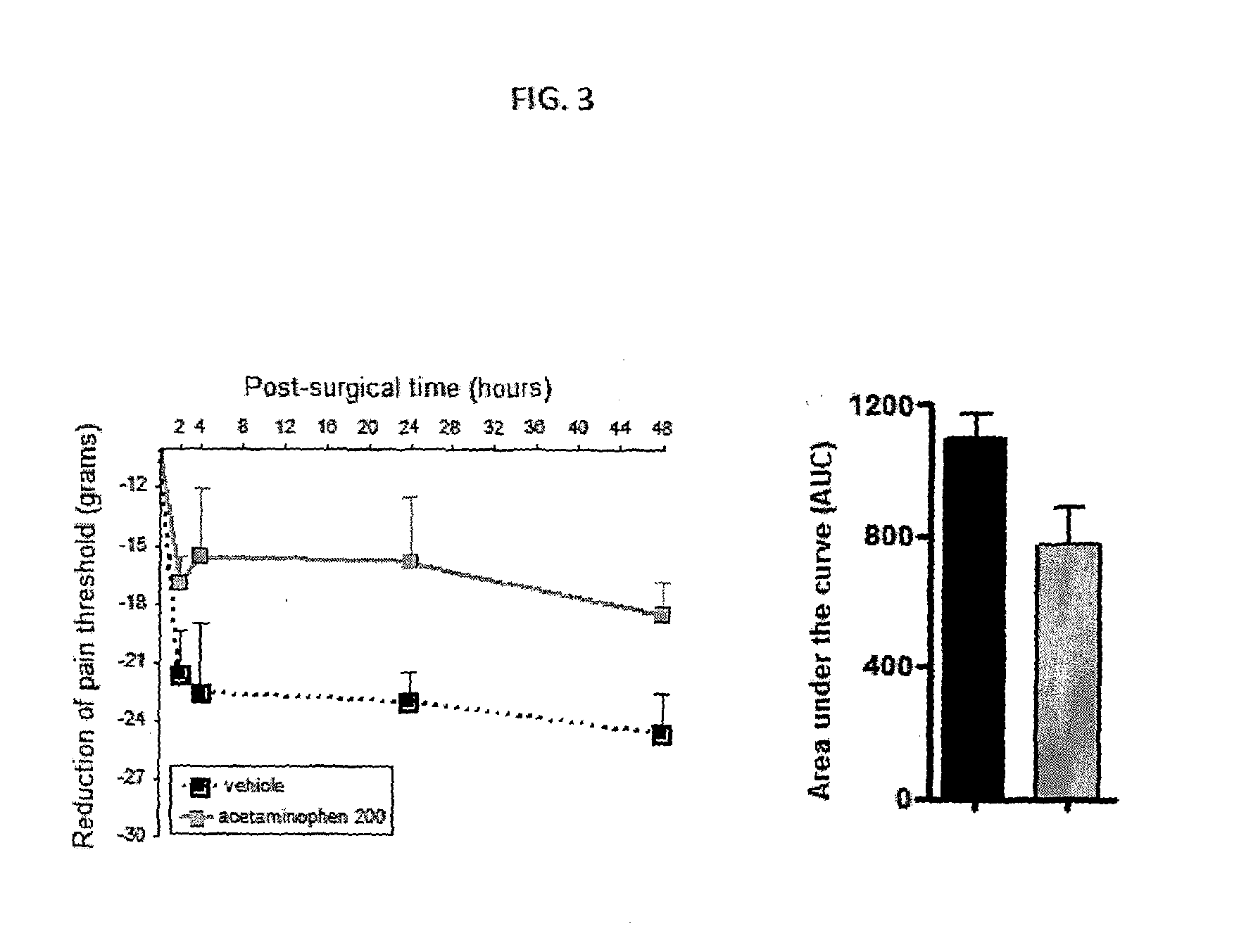
Injectable supersaturated acetaminophen solution for spinal administration
a technology of acetaminophen and injection solution, which is applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of limited risk of neurological damage, reversible loss of sensitivity, and no specific application found in the field of analgesic therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0116]Acetaminophen supersaturated solution injectable by spinal administration having the following formulation:
acetaminophen15 mginjectable sterile water 1 ml
[0117]The solution had a pH of 5.7
example 2
[0118]Acetaminophen supersaturated buffered solution injectable by spinal administration having the following formulation:
acetaminophen 20 mginjectable sterile water 1 mlcitric acid0.45 mgsodium dihydrogen phosphate0.91 mg
[0119]The pH of the solution was approximately 5.5
example 3
[0120]Buffered acetaminophen supersaturated solution injectable by spinal administration having the following formulation:
acetaminophen 80 mginjectable sterile water 1 mlcitric acid0.45 mgsodium dihydrogen phosphate0.91 mg
[0121]The pH of the solution was approximately 5.5
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com