Compositions and methods for the treatment of wounds, disorders, and diseases of the skin
a technology for disorders, wounds, and skin, applied in the direction of drug compositions, enzymology, viruses, etc., can solve the problems of difficult to chew and swallow food, chronic malnutrition, and excessive scarring, and achieve the effects of increasing, increasing, and/or supplementing the formation of hydroxylysine residues, enhancing, increasing, and/or supplementing the formation of anchoring fibrils of subjects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0158]A pharmaceutical composition comprising:
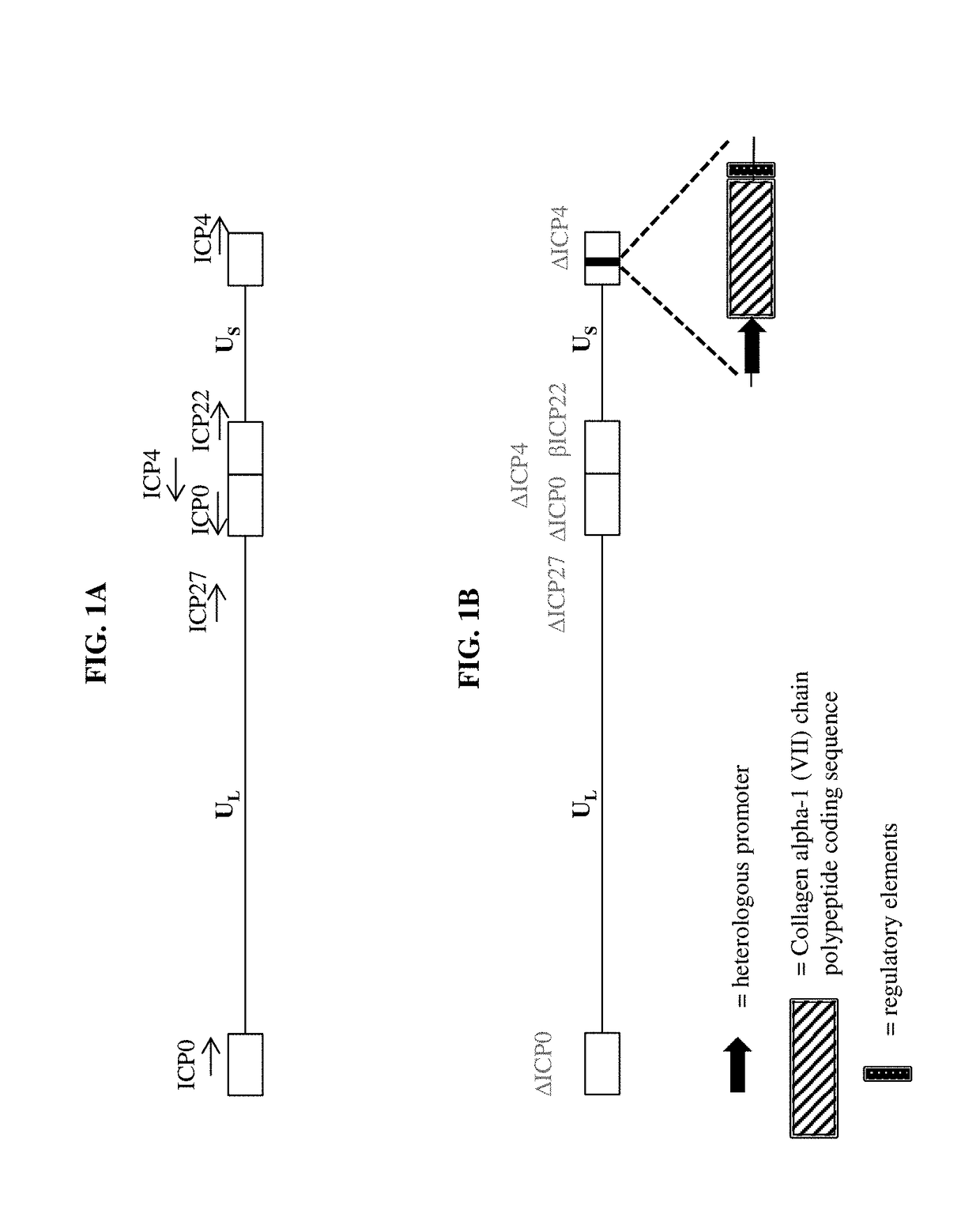
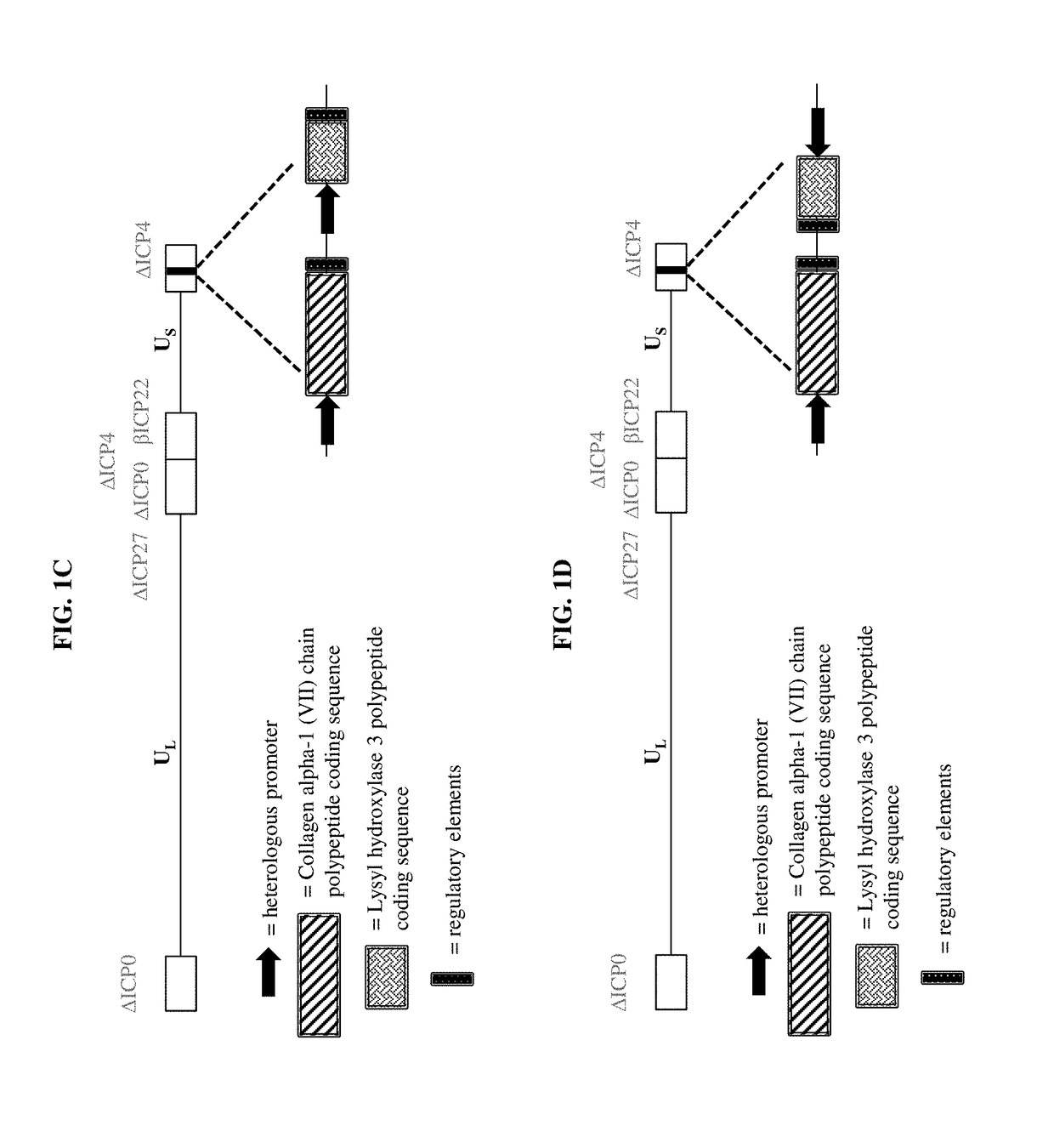
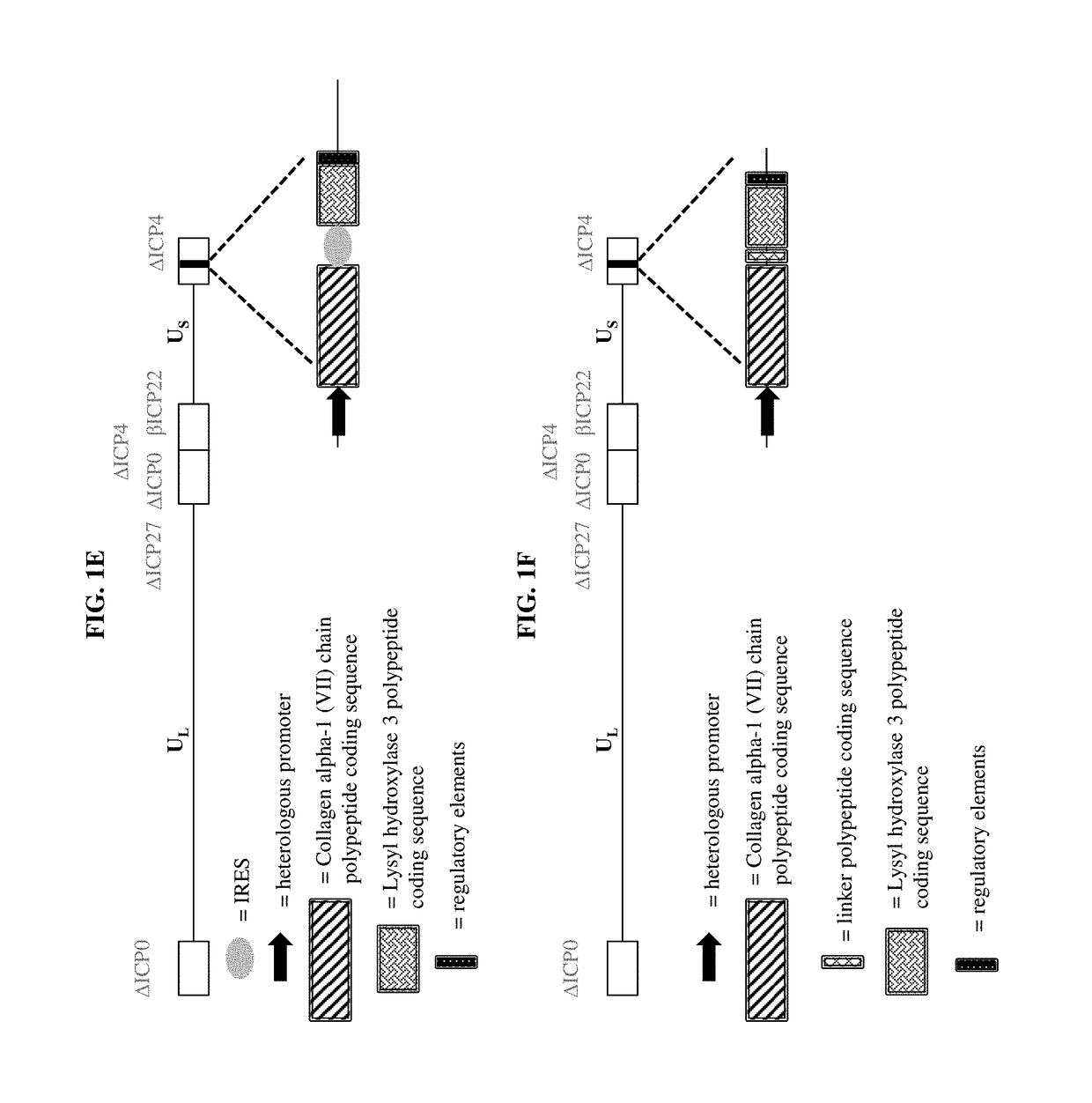
[0159]a) a virus comprising a vector, wherein the vector comprises one or more transgenes encoding a polypeptide selected from the group consisting of a Collagen alpha-1 (VII) chain polypeptide, a Lysyl hydroxylase 3 polypeptide, and a chimeric polypeptide thereof; and
[0160]b) a pharmaceutically acceptable carrier.
embodiment 2
[0161]The pharmaceutical composition of embodiment 1, wherein the virus is an adenovirus, adeno-associated virus, retrovirus, lentivirus, sendai virus, herpes simplex virus, vaccinia virus, or any hybrid virus thereof.
embodiment 3
[0162]The pharmaceutical composition of embodiment 1, wherein the virus is a herpes simplex virus (HSV).
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com