Method of Enhancing Delivery of Therapeutic Compounds to the Eye
a technology of therapeutic compounds and eye, which is applied in the direction of algae medical ingredients, peptide/protein ingredients, drug compositions, etc., can solve the problems of limited delivery effect of current methods, such as intravitreal injection or implanted drug delivery devices, and achieve the effects of improving or restoring light sensitivity, improving or restoring vision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ncreases Delivery of AAV2-Vector Encoding Chop2 to the Retina
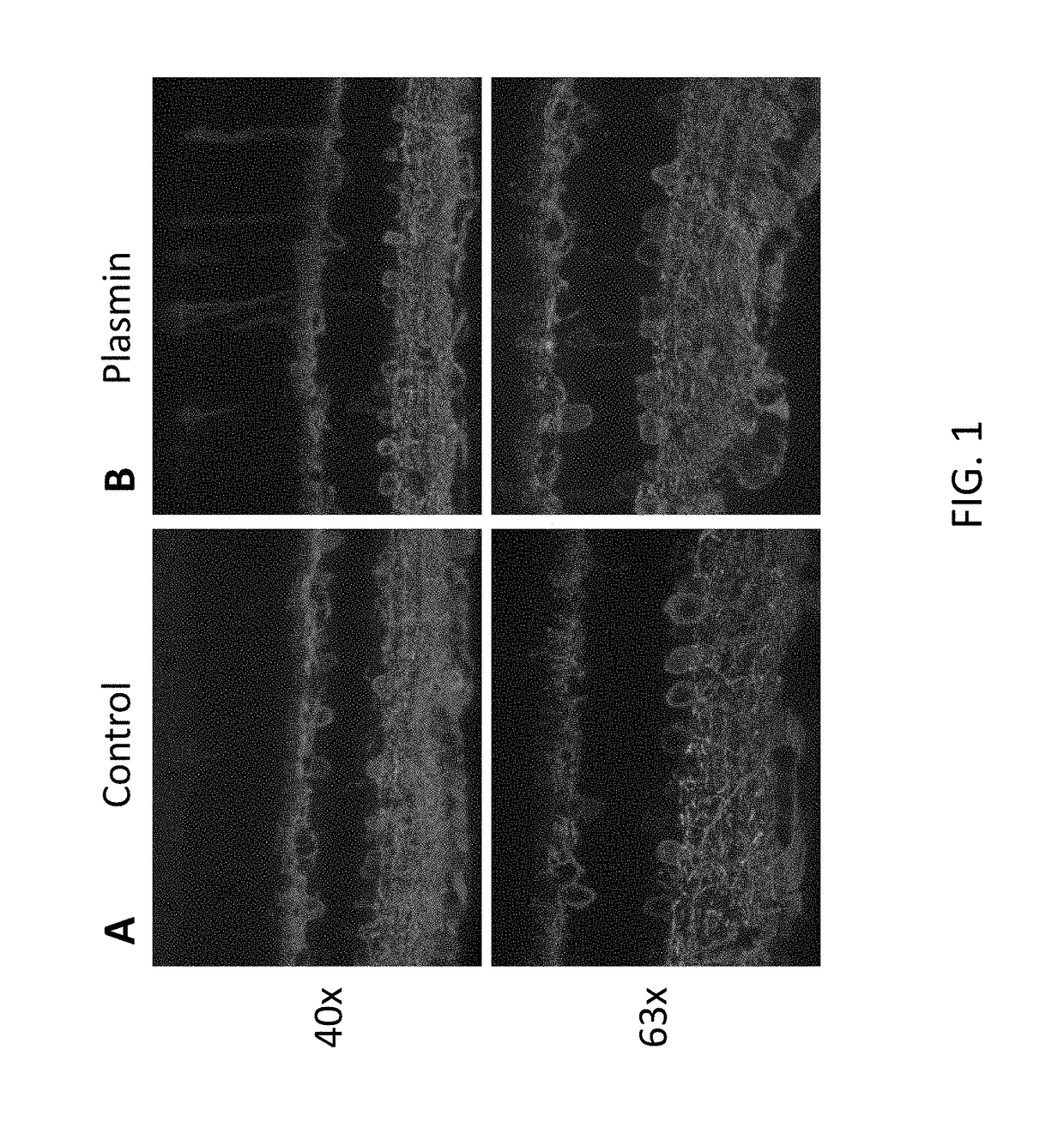
[0105]Delivery of a therapeutic viral construct encoding a functional GFP-channelopsin-2 protein to the retina was examined. Injection of 6×1012 vg / ml of AAV2 vector AAV2 / 2-ChR2-GFP-WPRE-hGHpA in control (FIG. 1A) or co-injection with plasmin (FIG. 1B) was performed into the vitreous space of the eye of one month old C56BL / 6J. The concentration of plasmin injected with vector was 0.025IU / eye. After one month, the mice retinas were isolated, and retinal vertical sections were prepared. The sections were immunostained and cells were counted. Immunofluorescence analysis of the sections showed that co-injection with plasmin increased the transduction efficiency of the therapeutic AAV2-ChR2-GFP vector, as evidenced by increased fluorescence in comparison to control (FIG. 1).
example 2
ncreases Transduction Efficiency in Retinal Ganglion Cells
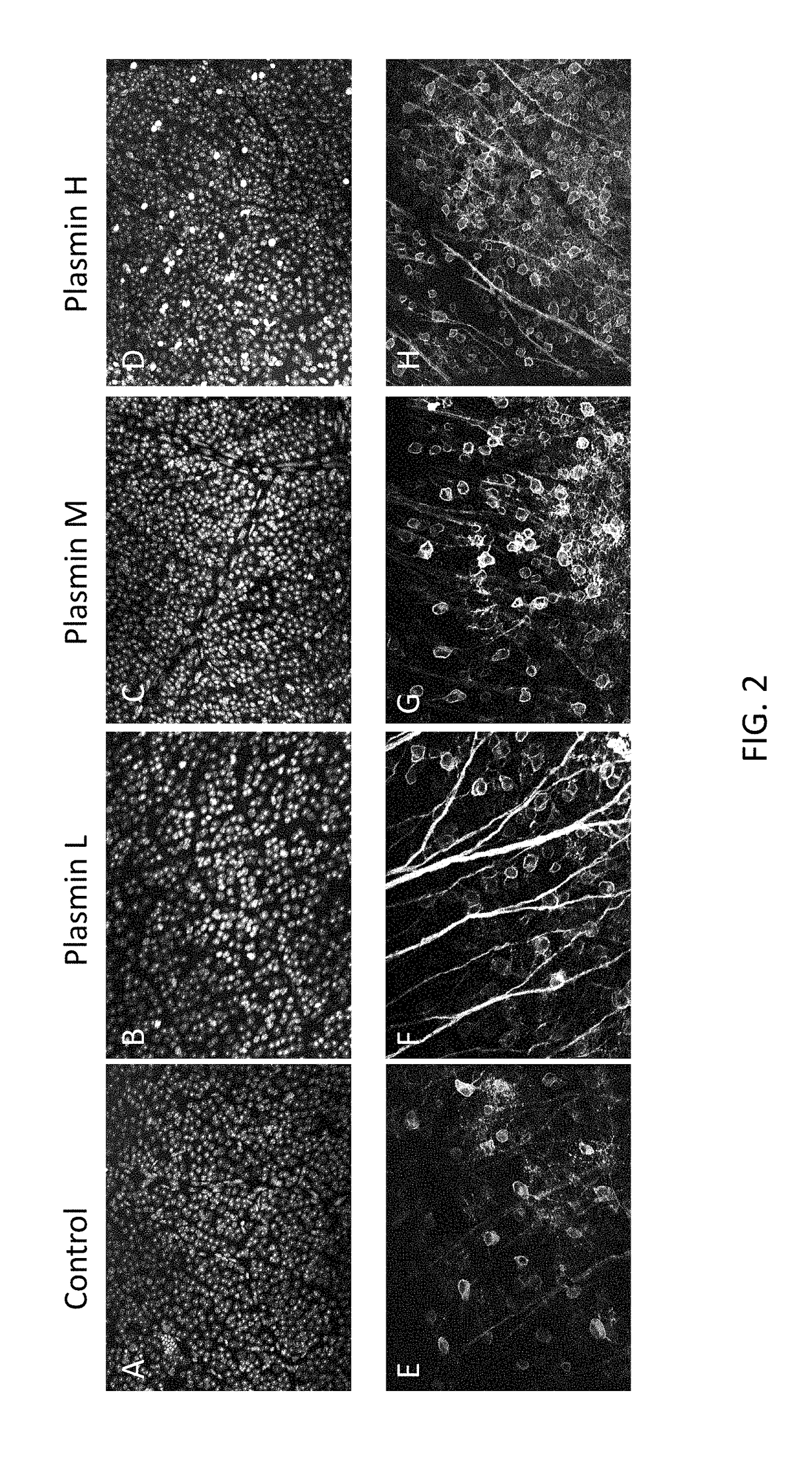
[0106]Using the same experimental set-up as in Example 1, a vector (2×1012 vg / ml) encoding GFP was co-injected with either control or varying concentrations of plasmin: low (L=0.005 IU / eye), middle (M=0.025IU / eye), and high (H=0.100IU / eye). After 1 month, retinal whole mounts were prepared and immunostained. Representative images for each plasmin concentration and control are shown in FIG. 2. As shown, treatment with plasmin increases GFP expression.
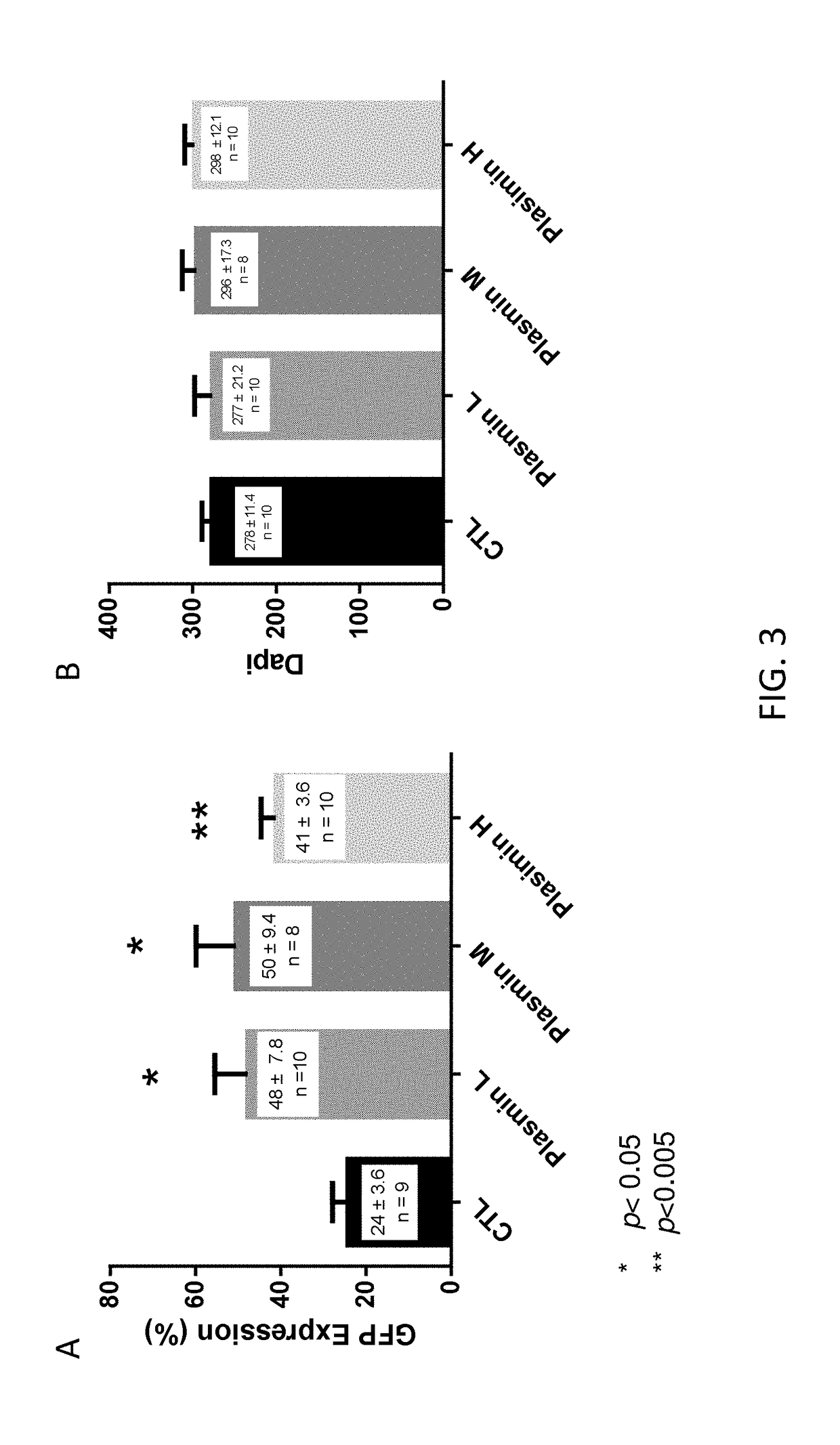
[0107]To further quantify these results, GFP-expressing retinal ganglion cells were counted from multiple unit areas of 223 μm×167 μm. The results are presented in FIG. 3A. As shown, treatment with low, middle and high doses of plasmin resulted in statistically significantly increased levels of GFP expression in retinal ganglion cells.
[0108]Neurotoxicity as a result of plasmin injection was also examined. The retinal whole mounts were stained with DAPI for cell-counting. The numbe...
example 3
ncreases Transduction Efficiency in Retinal Bipolar Cells
[0109]Comparison of the transduction efficiency of a viral vector encoding mCherry fluorescent protein when co-injected with different concentrations of plasmin was assessed in vivo. Specifically, overall levels and the localization of mCherry expression throughout the retina were examined. An AAV2 vector with an Y444F capsid mutation carrying mCherry under control of an mGluR6 promoter were injected at a concentration of 2×1012 vg / ml. The mGluR6 promoter directs expression of mCherry specifically to the retinal bipolar cells.
[0110]The AAV2 mCherry vector was co-injected with three doses of plasmin, high (H=0.100IU / eye), middle (M=0.025IU / eye), and low (L=0.005IU / eye). After 1 month, the retinas were isolated and retinal whole-mounts were prepared. Transduction efficiency was evaluated by immunostaining of mCherry for immunofluorescence analysis and cell counting. Cells were counted from multiple unit areas of 223 μm×167 μm.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com