Use of glucocorticoid receptor antagonist and somatostatin analogues to treat acth-secreting tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of a Subject with an Ectopic ACTH-Secreting Tumor
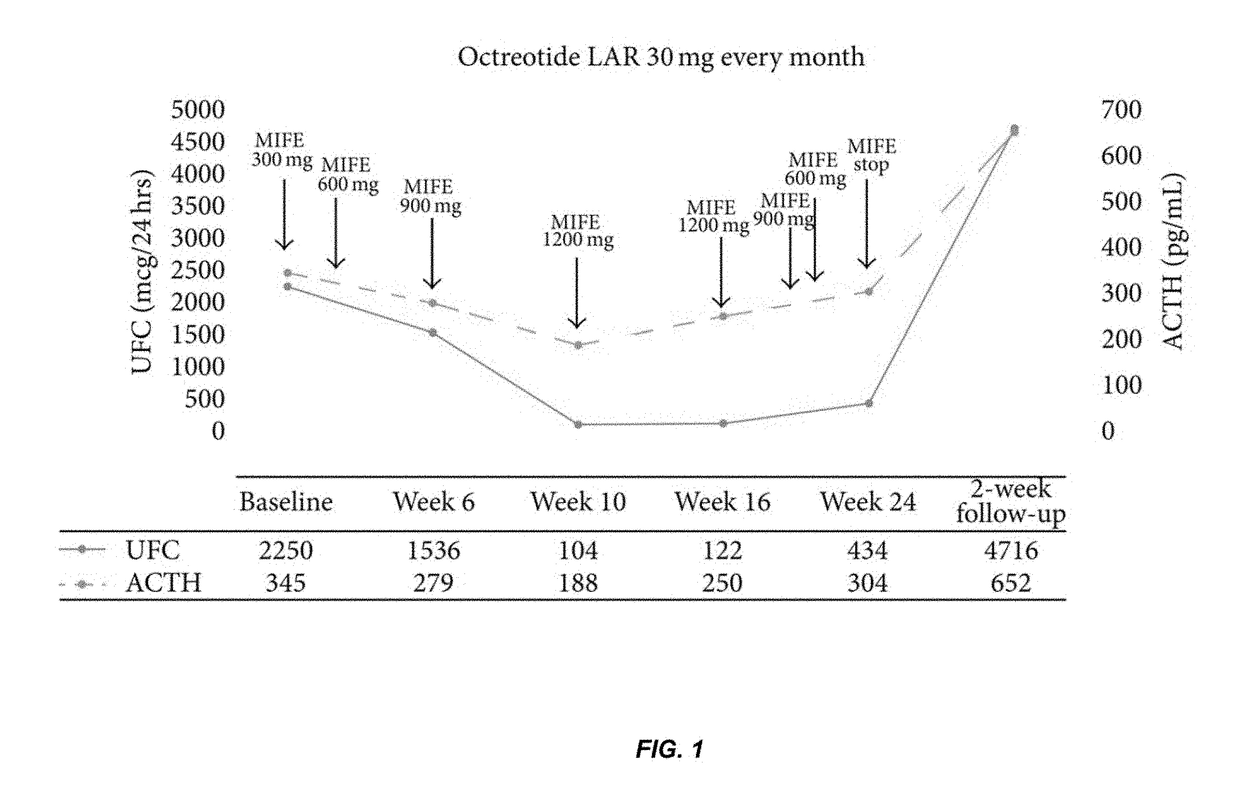
[0188]A human patient with an ectopic ACTH-secreting pancreatic neuroendocrine tumor metastatic to liver gastrinoma presented with symptoms of ectopic Cushing's Syndrome. The patient was treated with the maximum recommended dose of octreotide long-acting release (LAR), a partial biochemical response was noted (ACTH decreased from 517 pg / mL (113.7 pmol / L) to 345 pg / mL (75.9 pmol / L)), but the Cushing's symptoms were not controlled. After three months of therapy with octreotide LAR, the patient was enrolled in a 24-week, phase 3 clinical trial of mifepristone (MIFE) for inoperable hypercortisolemia.
[0189]Prior to the start of MIFE, baseline urinary-free cortisol (UFC) was 2250 mcg / 24 hours (6207 nmol / 24 hours) and ACTH was 345 pg / mL (75.9 pmol / L). Late-night salivary cortisol (1.71 mcg / dL (47.2 nmol / L)) and serum cortisol (46 mcg / dL (1256 nmol / L)) were also elevated (Table 1). At the time of enrollment, the patient had overtly cushingoid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Current | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com