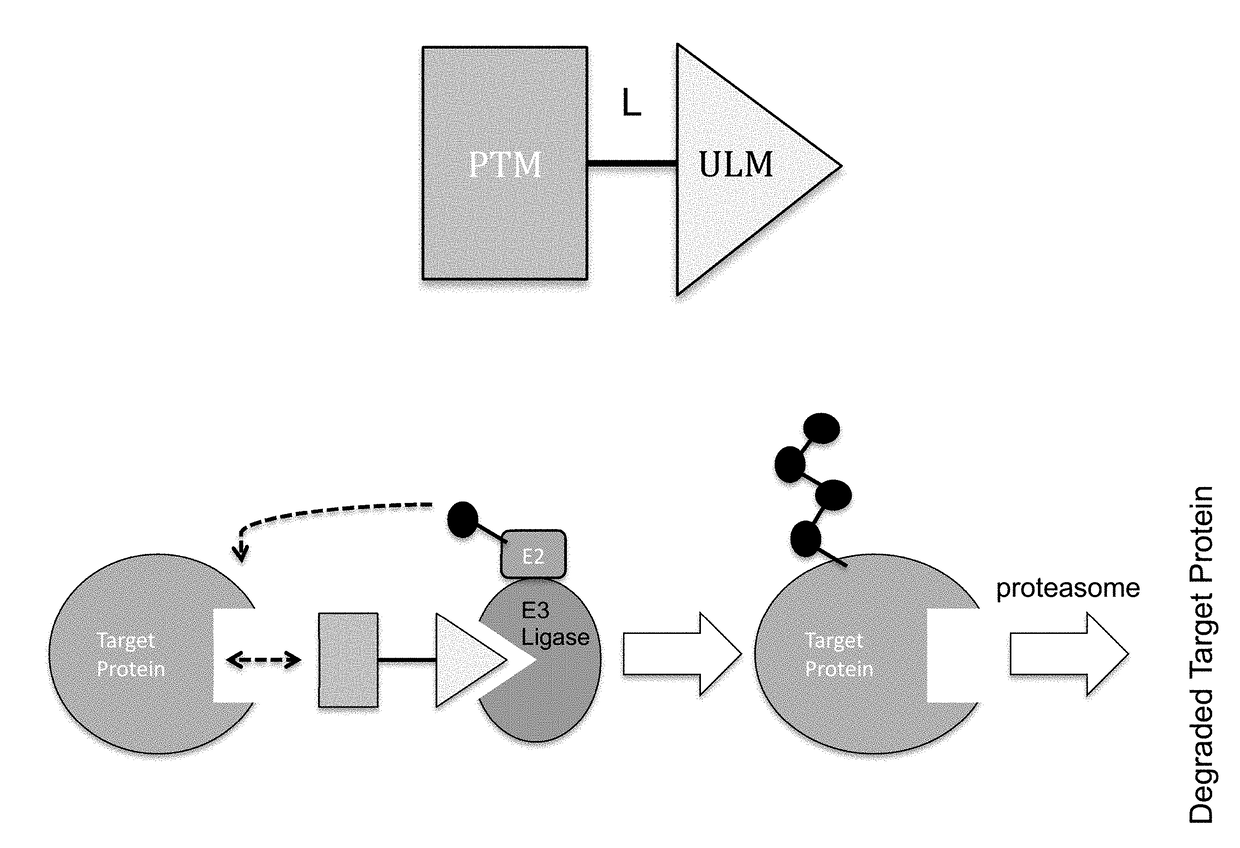
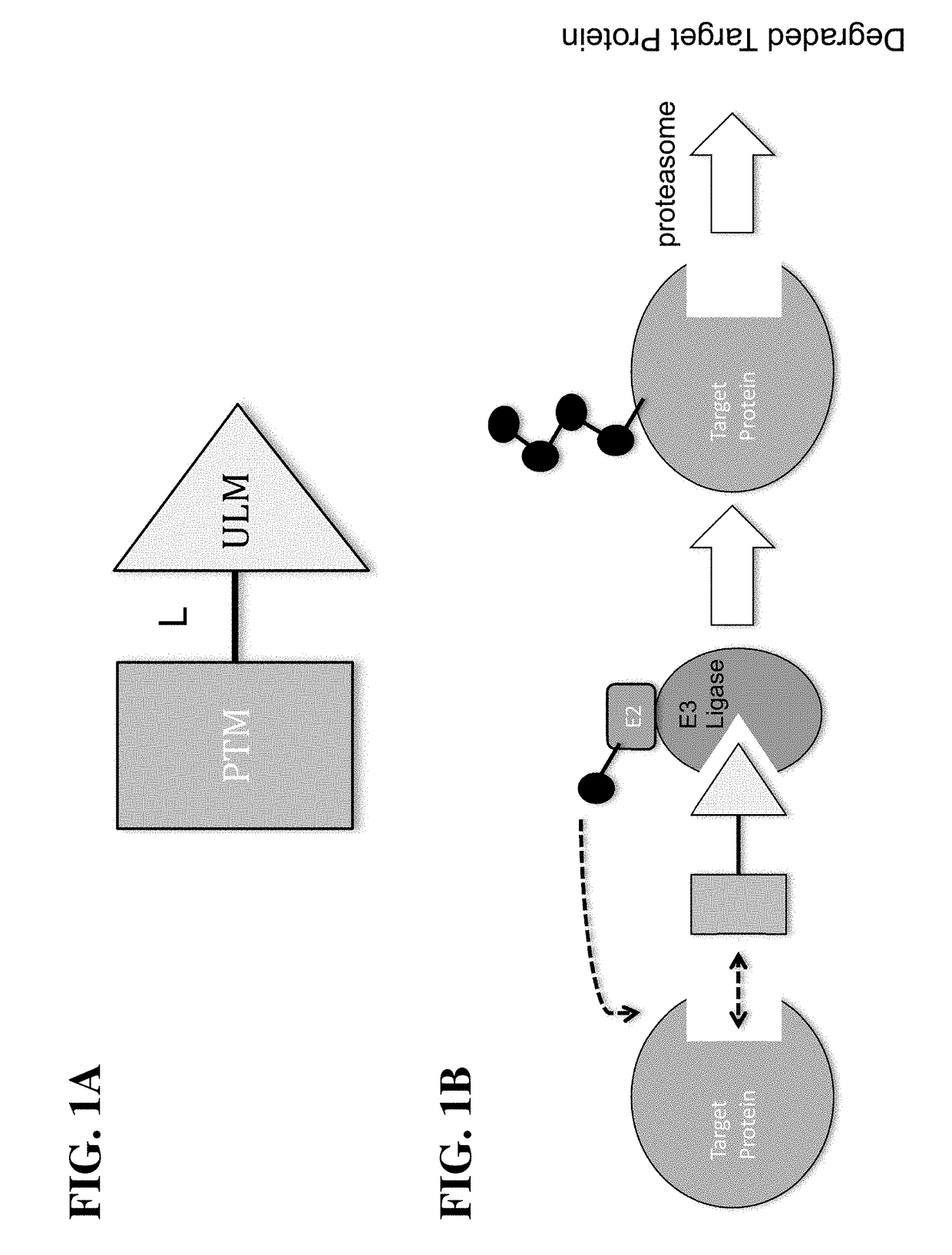
Cereblon ligands and bifunctional compounds comprising the same
a technology of ligands and e3 ligases, applied in the field ofimide-based compounds, can solve the problems of difficult development of ligands of e3 ligases, difficult targeting of protein-protein interactions using small molecules, and encouraging the effect of brd4 inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Abbreviations
[1602]ACN: acetonitrile[1603]ADDP: 1,1′-(azodicarbonyl)dipiperidine[1604]BAST: N,N-bis(2-methoxyethyl)aminosulfur trifluoride[1605]BPO: benzoyl peroxide[1606]Cbz: Carbonylbezyloxy[1607]DAST: diethylaminosulfur trifluoride[1608]DBE: 1,2-dibromoethane[1609]DCM: dichloromethane[1610]DEAD: diethyl azodicarboxylate[1611]DIAD: diisopropyl azodicarboxylate[1612]DIBAL: disiobutylaluminium hydride[1613]DIEA or DIPEA: diisopropylethylamine[1614]DMA: N,N-dimethylacetamide[1615]DMF: N,N-dimethylformamide[1616]DMP: Dess-Martin periodinane[1617]EA: ethyl acetate[1618]EDCI: 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide[1619]HBTU: N,N,N′N′-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate[1620]HMDS: bis9trimethylsilyl)amine[1621]HMPA: hexamethylphosphoramide[1622]LDA: lithium diisopropylamide[1623]MCPBA: meta-chloroperoxybenzoic acid[1624]MsCl: methanesulfonyl chloride[1625]M.W: microwave[1626]NBS: N-bromosuccinimide[1627]NMP: N-methylpyrrolidone[1628]PCC: pyridinium chlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemical structure | aaaaa | aaaaa |
| chemical structural unit | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com