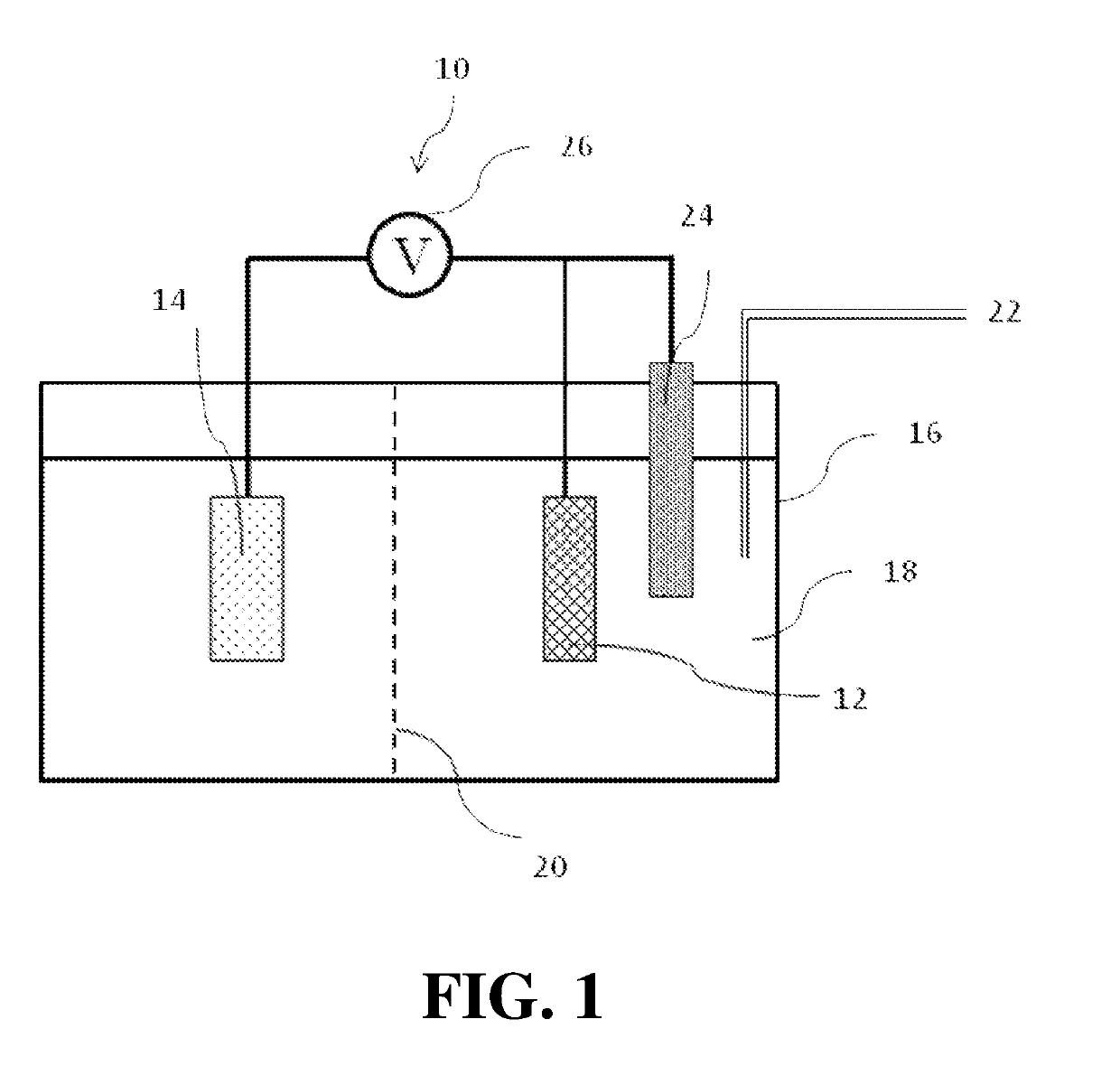
Electrochemical catalyst for conversion of co2 to ethanol
a technology of electrochemical catalyst and co2 conversion, which is applied in the field of electrochemical catalyst for converting carbon dioxide to ethanol, can solve the problems of limiting the yield of any one liquid product to single-digit percentages, unable to achieve efficient and efficient reduction of co2 /sub>into and unable to achieve efficient and efficient reduction of co2 /sub> into a desirable liquid fuel. , to achieve the effect of efficient and selective conversion of carbon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Carbon Nanospikes
[0053]The carbon nanospikes were grown on n-type 4-inch Si wafers 100 with As doping (2H2) and ammonia (NH3) at 650′C for 30 minutes. DC plasma was generated between the wafer (cathode) and the showerhead (anode) in a continuous stream of C2H2 and NH3 gas, flowing at 80 sccm and 100 sccm, respectively. The total pressure was maintained at 6 Torr with a plasma power of 240 W.
[0054]The carbon nanospikes were characterized as a dense nanotextured carbon film terminated by randomly oriented nanospikes approximately 50-80 nm in length, where each nanospike consists of layers of puckered carbon ending in a ˜2 nm wide curled tip. Raman spectra indicated that carbon nanospikes have similar structure to disordered, multilayer graphene. XPS indicated nitrogen doping density as 5.1±0.2 atomic %, with proportions of pyridinic, pyrrolic (or piperidinic) and graphitic nitrogens of 26, 25 and 37% respectively, with the balance being oxidized nitrogen.
example 2
Preparation of Cu / CNS Electrocatalyst
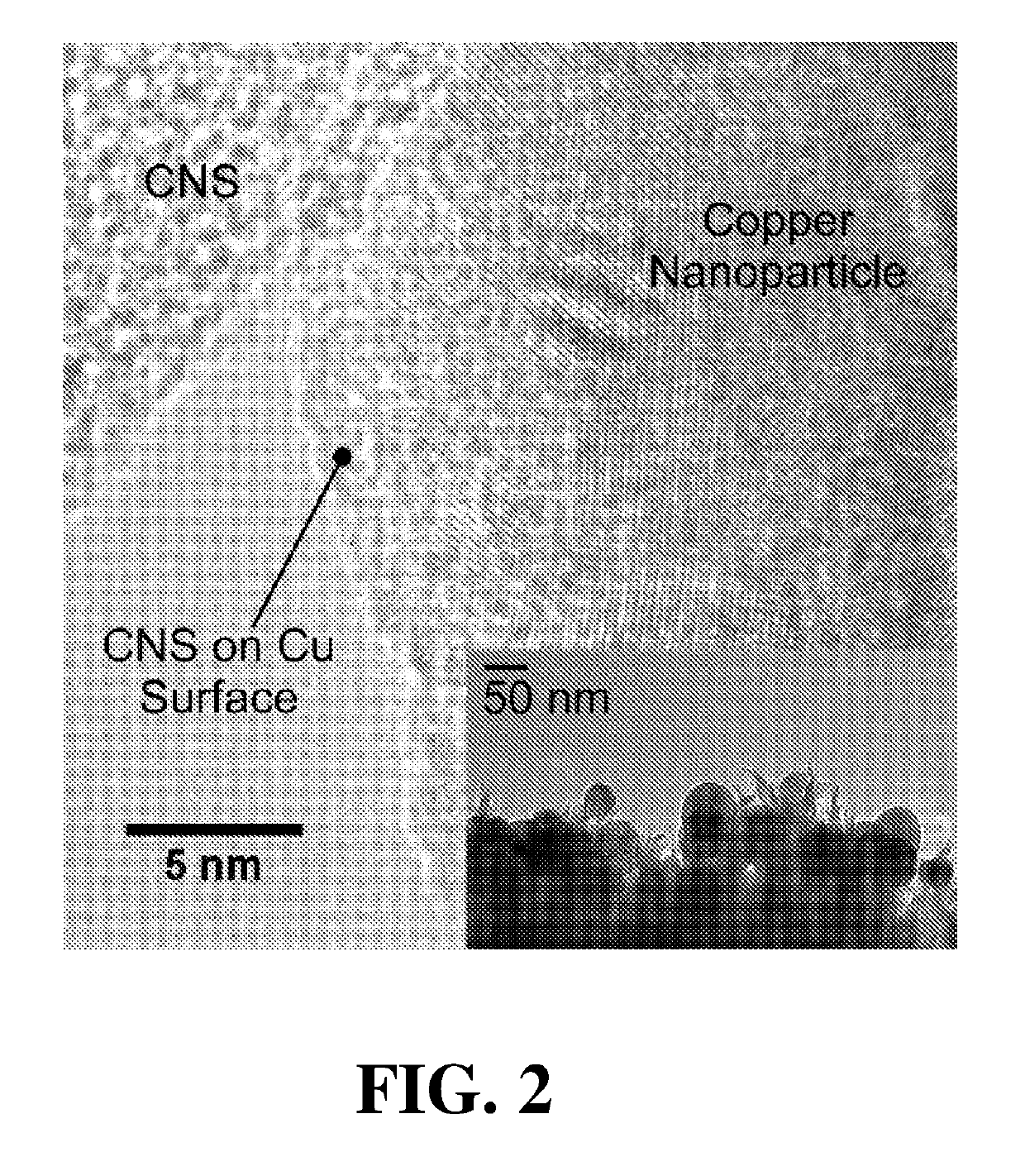
[0055]Cu nanoparticles were electronucleated from CuSO4 directly onto carbon nanospikes, and imaged via SEM. These well-dispersed Cu nanoparticles have sizes ranging from about 30 nm to 100 nm with average size of 39 nm, with a density ca. 1.2×1010 particles cm−2. According to the average particle size, the coverage of Cu on carbon nanospikes is ca. 14.2%. High-resolution TEM on scraped samples (HR-TEM), as provided in FIG. 2, illustrates the Cu nanoparticle and carbon nanospike interface, which indicates a close proximity between Cu nanoparticles and the carbon nanospikes. A lower magnification TEM image (FIG. 2, inset) confirms the particle size observed via SEM. The lattice spacing of this representative copper nanoparticle was measured as 0.204 nm, which is consistent with copper. A Cu2O composition with lattice spacing ca. 0.235 nm was present on surfaces of the copper nanoparticles, likely resulting from exposure to air during sample prepar...
example 3
Stability of Cu / CNS Electrocatalyst
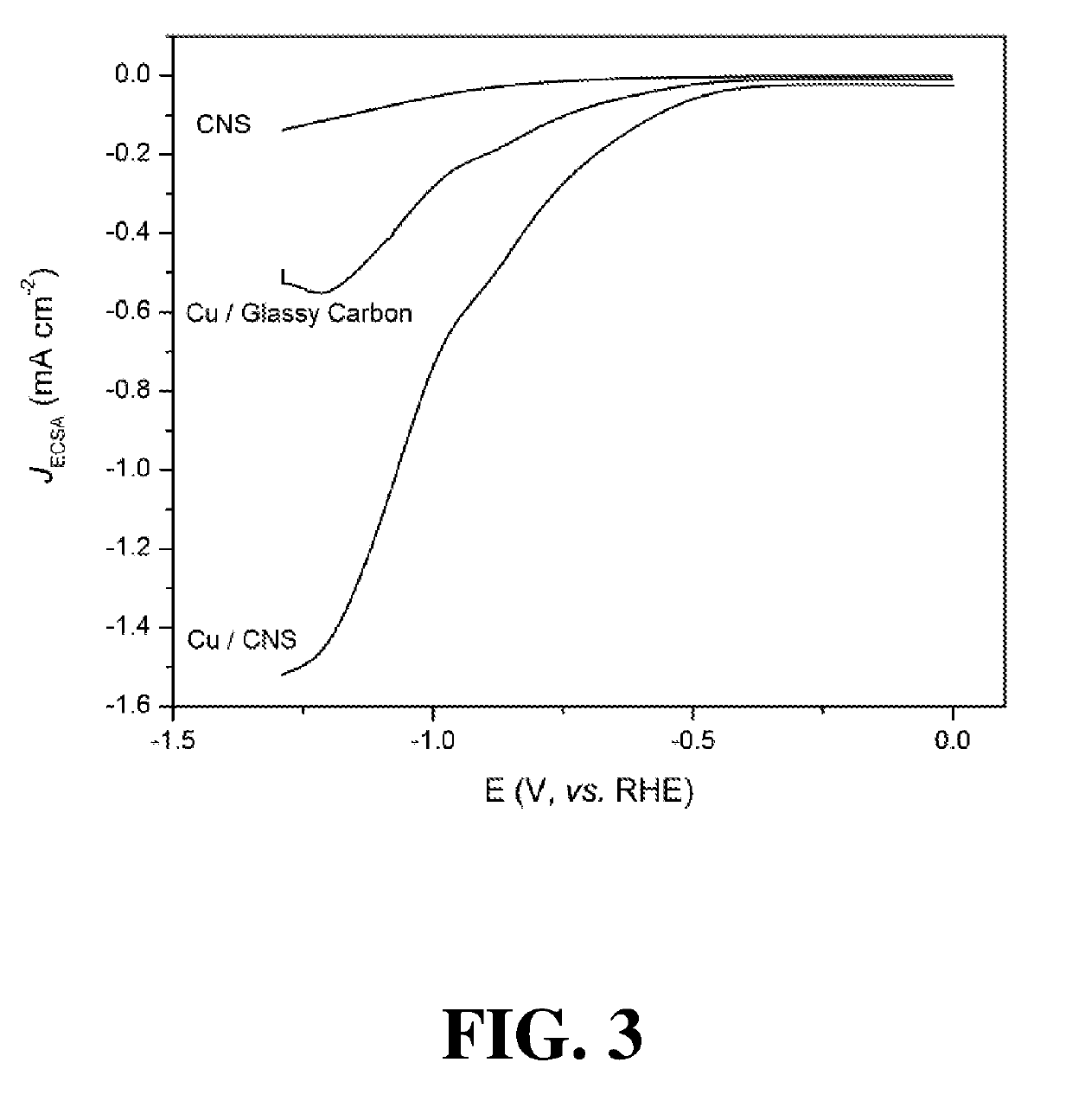
[0056]To investigate the short-term stability of the Cu / CNS electrocatalyst, additional HR-TEM images and EELS spectra were taken after a 6-hour CO2 reduction reaction, and no obvious changes were observed. Likewise, X-ray Photoelectric Spectroscopy (XPS) measurements for Cu 2p3 / 2 showed a similar asymmetric peak at 932 eV, which indicates that the Cu nanoparticles were stable after a 6-hour reaction and were mainly comprised of Cu0. However, after a 6-hour electroreduction, the fraction of graphitic-N significantly decreased (38.9 to 10.7%), while pyridinic-N and pyrrolic / amine-N increased (14.2 to 24.7% and 39.6 to 54.2%, respectively. While XPS cannot distinguish between pyrrole and amine, electroreduction from pyridinic-N to pyrrolic-N would require removal of a C atom; therefore, the increased pyrrolic / amine-N is likely piperidine, with no increase in pyrrolic fraction. No change in electrochemical activity was observed during this prolonged e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com