Modified L-Asparaginase
a technology of lasparaginase and l-asparaginase, which is applied in the field of lasparaginases, can solve the problems of inability to meet the needs of patients, etc., and achieves the effect of reducing the activity of pegylated biopharmaceuticals compared to unmodified biopharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
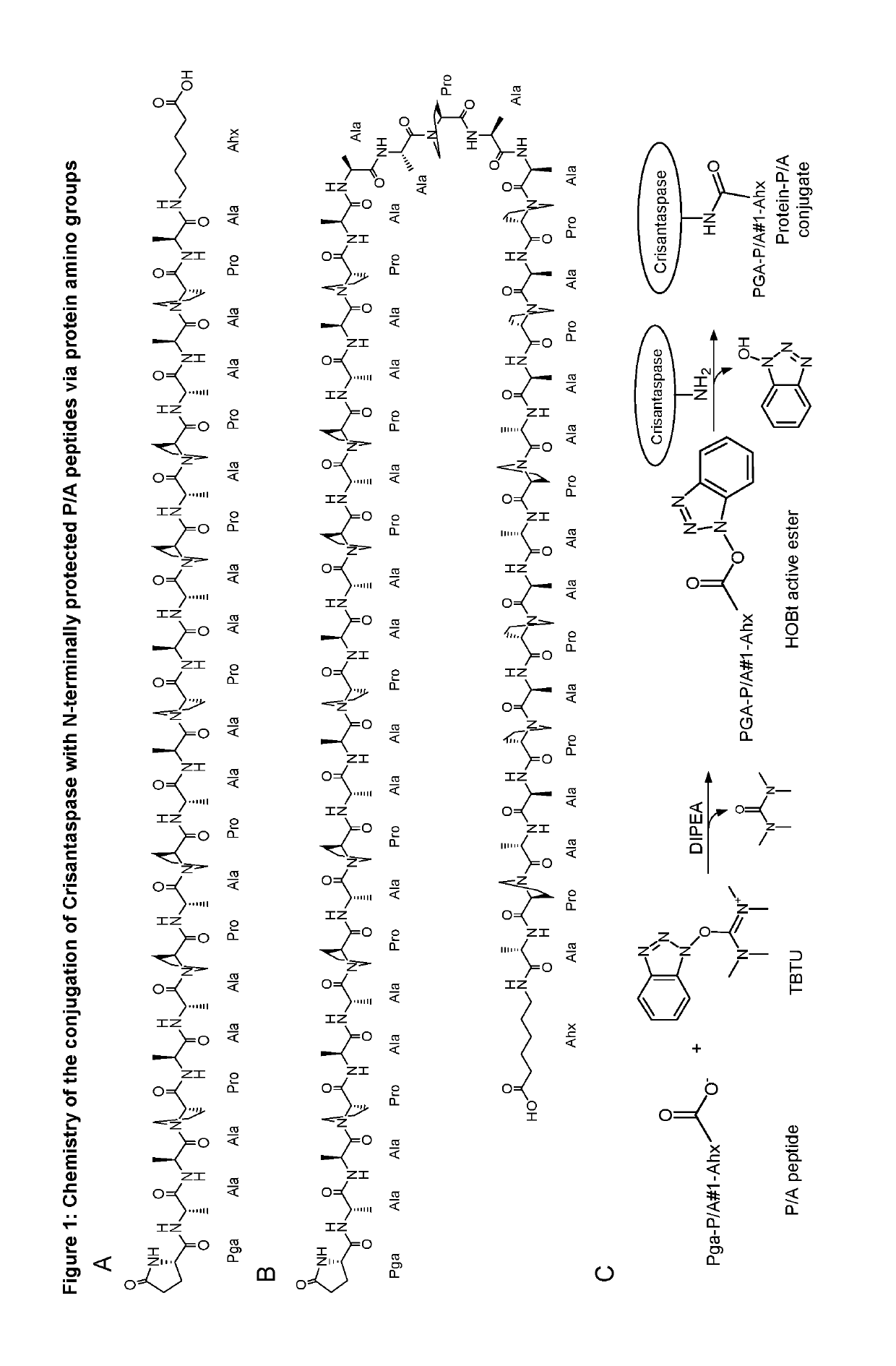
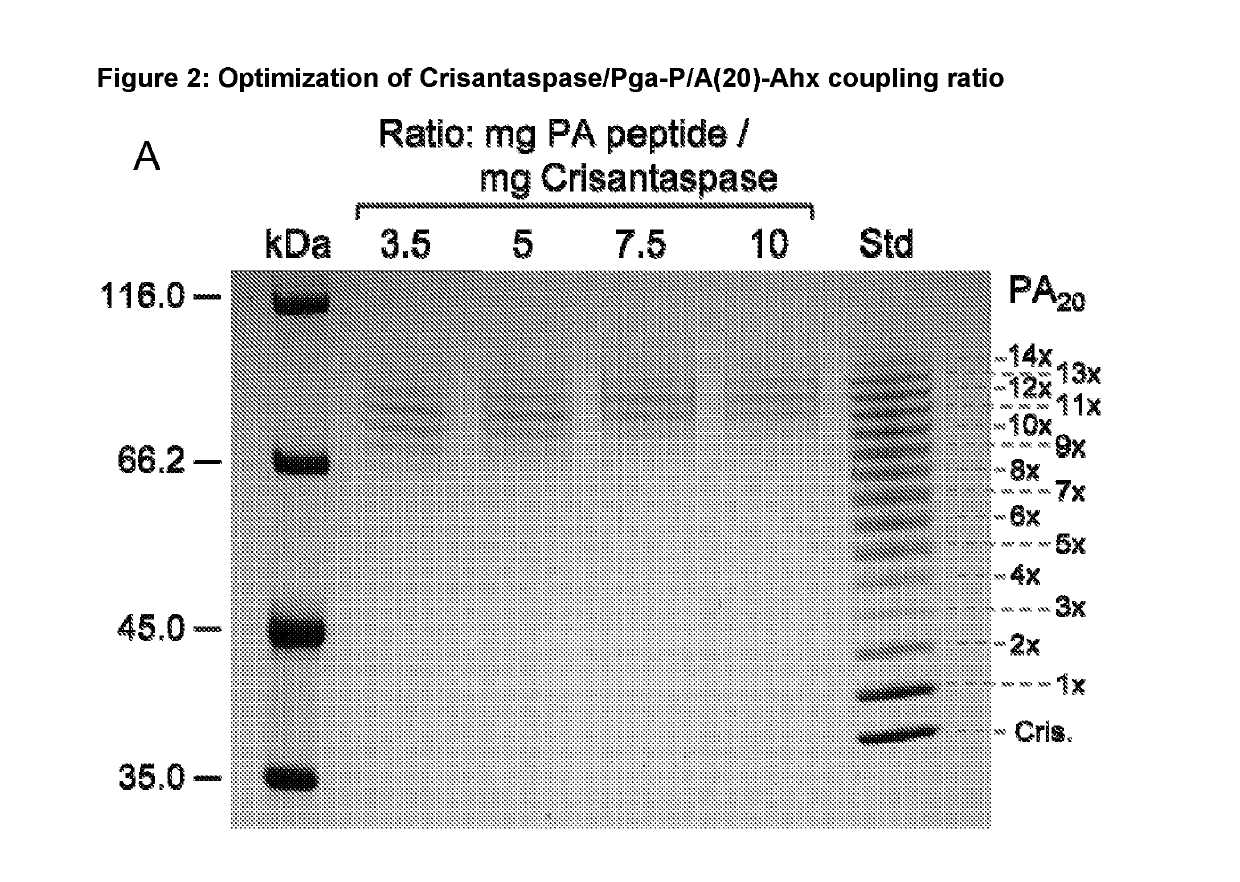
[0185]Optimization of Coupling Ratio for the Preparation of Pyroglutamoyl-P / A(20)-aminohexanoyl-Crisantaspase
[0186]4.38 mg Pga-P / A#1(20)-Ahx peptide (Part A of FIG. 1; TFA salt, purity 98%; PSL Peptide Specialty Laboratories, Heidelberg, Germany) (SEQ ID NO: 16, amino acid sequence shown in SEQ ID NO: 5) was dissolved in 66.3 μl DMSO. The chemical activation of the P / A peptide via its terminal carboxylate group was started by addition of 23.7 μL of a solution of 500 mM TBTU (CAS#125700-67-6; Iris Biotech, Marktredwitz, Germany) in DMSO and 2.7 μL DIPEA to the peptide solution and vortexing (cf. Part C of FIG. 1). In this setup, the concentration of the peptide was 25.8 mM and the molar ratio between DIPEA, TBTU and Pga-P / A#1(20)-Ahx was 5:5:1. After 10 min incubation at 25° C. the mixture was diluted in Eppendorf tubes according to Table 1.
[0187]A solution of Dickeya chrysanthemi L-Asparaginase (Crisantaspase, SEQ ID NO: 1, recombinant, produced in E. coli (lot RE-LAP-P57D) with a c...
example 2
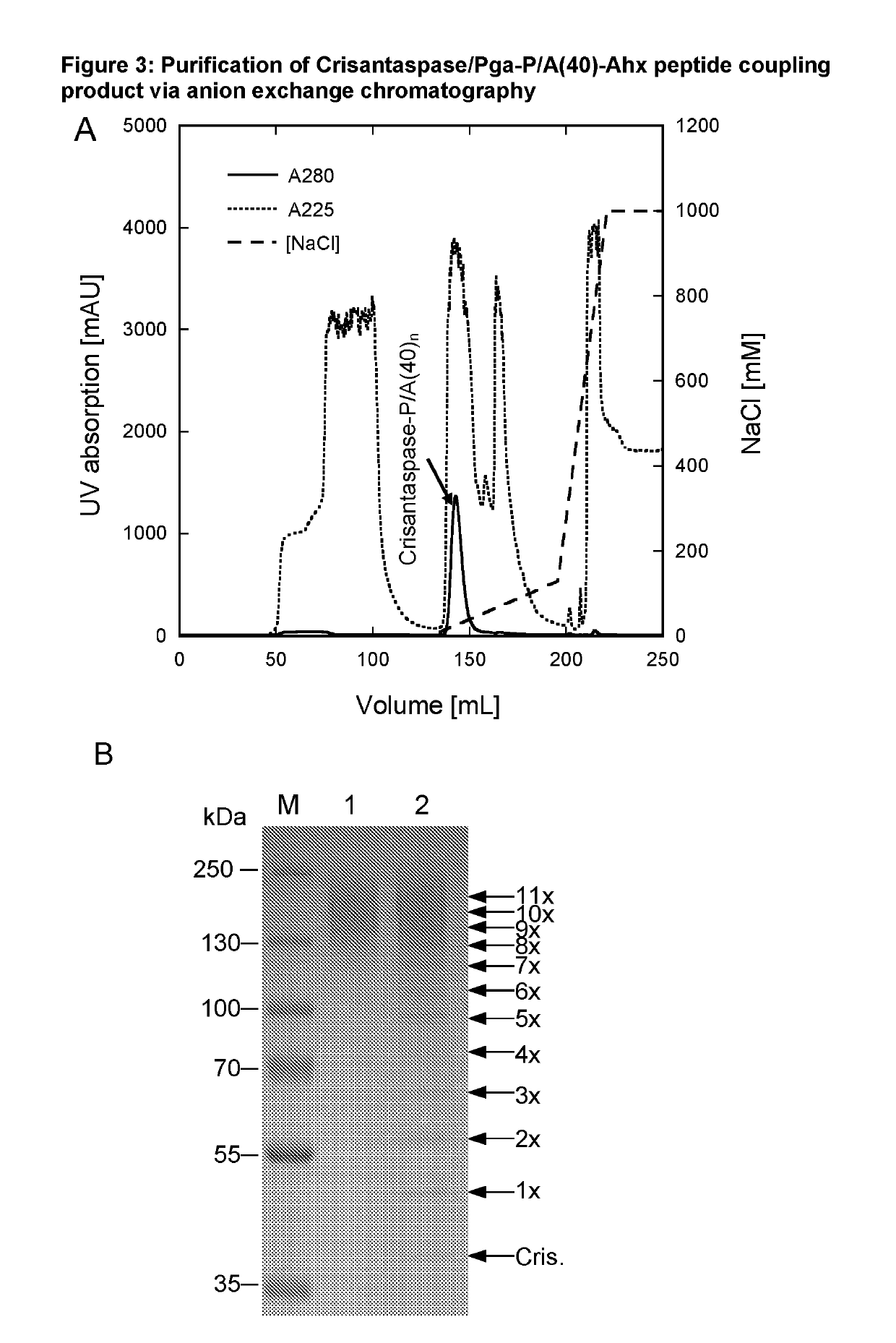
[0190]Preparation of Pyroglutamoyl-P / A(40)-aminohexanoyl-Crisantaspase
[0191]28 mg of the Pyroglutamoyl-P / A#1(40)-Ahx peptide (SEQ ID NO. 17, amino acid sequence shown in SEQ ID NO: 15), Part B of FIG. 1, TFA salt, purity 98%; Almac Group, Craigavon, UK) was dissolved in 1324 μL of anhydrous DMSO (99.9%; Sigma-Aldrich, Taufkirchen, Germany). To achieve chemical activation of the P / A peptide via its terminal carboxylate group, 162 μL of a solution of 500 mM TBTU (CAS# 125700-67-6; Iris Biotech, Marktredwitz, Germany) in DMSO and, after mixing, 14 μL DIPEA (99.5%, biotech. Grade, Sigma-Aldrich) were added. The whole mixture was vortexed briefly and incubated for 20 min at 25° C. (cf. Part C of FIG. 1). In this setup, the peptide concentration was 5.41 mM and the molar ratio between DIPEA, TBTU and Pga-P / A#1(40)-Ahx was 10:10:1.
[0192]3.5 mL of an ice-cold Crisantaspase solution (SEQ ID NO: 1)(2 mg / mL in PBS) was mixed with the activated peptide solution (1.5 mL), resulting in a mass rat...
example 3
[0194]Preparation of Pyroglutamoyl-P / A(20)-aminohexanoyl-Crisantaspase
[0195]21 mg of the Pyroglutamoyl-P / A#1(20)-Ahx peptide (SEQ ID NO: 5, Part A of FIG. 1; TFA salt, purity 98%; PSL Peptide Specialty Laboratories, Heidelberg, Germany) was dissolved in 1376 μL of anhydrous DMSO (99.9%; Sigma-Aldrich, Taufkirchen, Germany). To achieve chemical activation of the P / A peptide via its terminal carboxylate group, 114 μL of a solution of 500 mM TBTU (CAS# 125700-67-6; purchased from Iris Biotech, Marktredwitz, Germany) in DMSO and, after mixing, 10 μL DIPEA (99.5%, biotech. Grade, Sigma-Aldrich) were added. The whole mixture was vortexed briefly and incubated for 20 min at 25° C. (Part C of FIG. 1). In this setup, the peptide concentration was 7.58 mM and the molar ratio between DIPEA, TBTU and Pga-P / A#1(20)-Ahx was 5:5:1.
[0196]3.5 mL of an ice-cold Crisantaspase solution (SEQ ID NO: 1)(2 mg / mL in PBS) was mixed with the activated peptide solution (1.5 mL), resulting in a mass ratio betwe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com