Acinetobacter Y-3L-asparaginase gene as well as expression and application thereof
A technology of asparaginase and Y-3, applied in application, genetic engineering, plant gene improvement, etc., can solve the problems of cumbersome separation and purification methods, low yield, complicated extraction process, etc., and achieve the inhibition of acrylamide formation, high efficiency The effect of expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
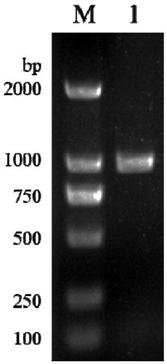
[0035] Acinetobacter ( Acinetobacter soli ) Cloning of Y-3 L-asparaginase gene
[0036] (1) Acinetobacter ( Acinetobacter soli ) Y-3 whole genome extraction: Acinetobacter Y-3 cells were collected by centrifugation, and the whole genome of the bacteria was extracted using the Bacterial DNA kit kit from OMGA Company.
[0037] (2) L-asparaginase primer design: according to the NCBI database Acinetobacter soli AsAase gene sequence in the whole genome nucleic acid sequence, design PCR upstream and downstream primers F1, R1, F2, R2 of L-asparaginase gene:
[0038] F1: 5'C GAGCTC ATGAATAAAATTGCCTTAATTT 3'( Sac I)
[0039] R1: 5'CCG CTCGAG TTAATCCTCAGCCTTAATGGTGG 3'( xho I)
[0040] F2: 5'TGCAAAAGCCGCAGC AGATCT ATGAATAAAATTGCCTT 3'( Bgl II)
[0041] R2: 5’GAATTCGAGCTCCCG GGTACC TTAATCCTCAGCCTTA 3'( Kpn I)
[0042] (3) Cloning of L-asparaginase gene: Use the whole genome of Acinetobacter Y-3 as a template, and use the primers designed above for PCR amplification....
Embodiment 2
[0044] Example 2: Expression and purification of recombinant L-asparaginase in Escherichia coli
[0045] (1) Expression vector construction: use pMD19-T-AsAase and vector pET30a(+) with Sac and xho Double enzyme digestion at 37°C for 9 hours. Use the gel extraction kit to recover the target fragment, T 4 Enzyme ligated overnight at 16°C, transformed into competent cells E. coliDH5α was spread on a kanamycin (Kana) resistant plate, and the positive transformants were picked for enrichment, and the extracted plasmid was named pET30a(+)-AsAase. Transform the plasmid pET30a(+)-AsAase into competent cells E. coli BL21(DE3), spread on Kana resistance plate, pick positive transformants for sequencing.
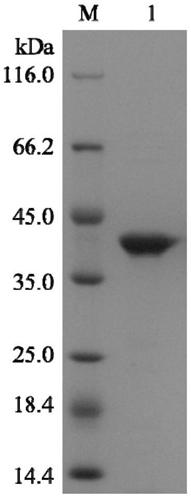
[0046] (2) Expression of recombinant L-asparaginase: the recombinant bacteria were inoculated in LB liquid medium containing 100 μg / mL Kana, and cultured overnight at 37°C and 180 rpm to make seed liquid. Then transfer 1% of the inoculum to the corresponding 100ml LB ...
Embodiment 3
[0050] Embodiment 3: Expression and purification of recombinant L-asparaginase in Bacillus subtilis
[0051] (1) Expression vector construction: use pMD19-T-AsAase and vector PCBS345 with Bgl II and Kpn Double enzyme digestion at 37°C for 9 hours. Use the gel extraction kit to recover the target fragment, T 4 Enzyme ligated overnight at 16°C, transformed into competent cells E. coli DH5α was spread on the resistance plate, and the positive transformants were picked to multiply, and the extracted plasmid was named PCBS345-AsAase. Transformation of plasmid PCBS345-AsAase into competent cells B. subtilis 001, pick positive transformants for sequencing.
[0052] (2) Expression of recombinant L-asparaginase: the recombinant bacteria were inoculated in the fermentation medium and cultured at 37°C and 180 rpm for 24-48h.
[0053] (3) Purification of recombinant L-asparaginase: the cells were collected by centrifugation at low temperature, and the supernatant was the crud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com