System and method for characterizing drug product impurities
a drug product and impurity detection technology, applied in the field of protein separation methods and cell culture methods, can solve the problems of overestimation of minor lmw species, inability to unambiguously confirm the proposed structure resulting from these methods at the intact protein level, and compromise both drug efficacy and safety, so as to reduce the level of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
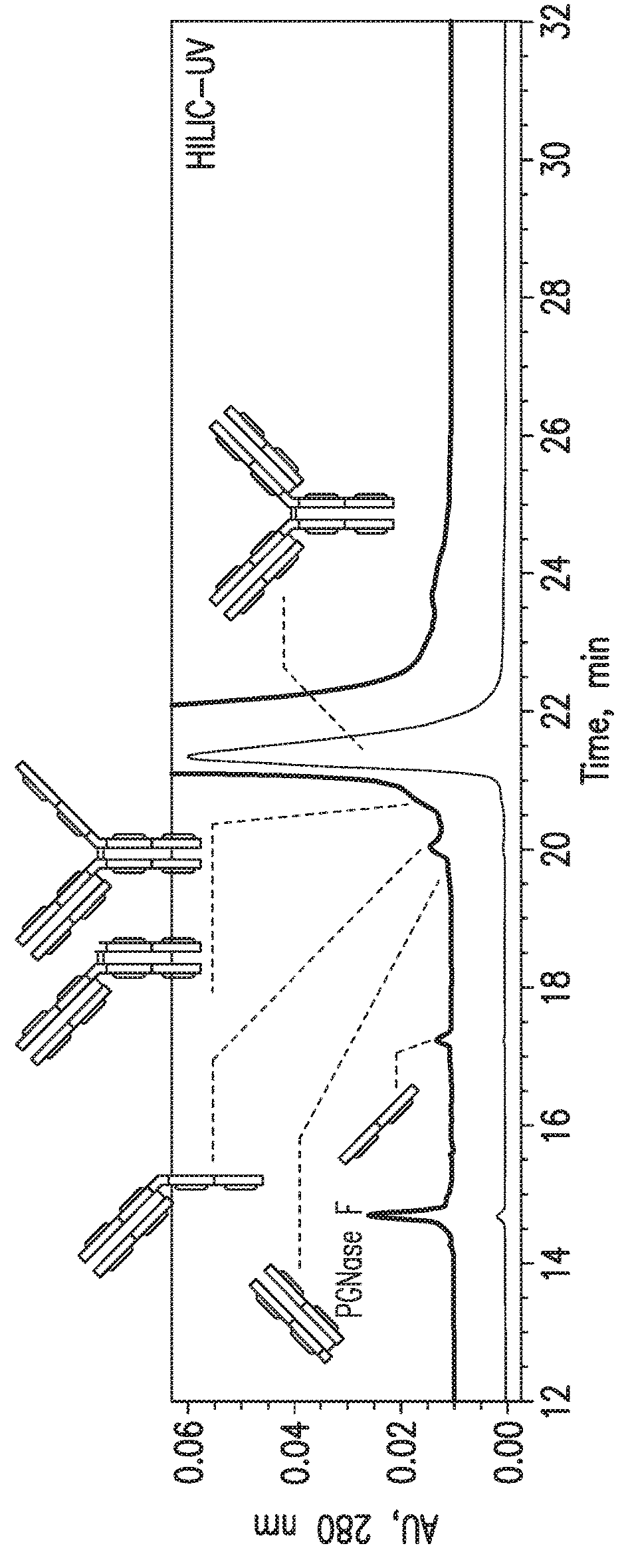
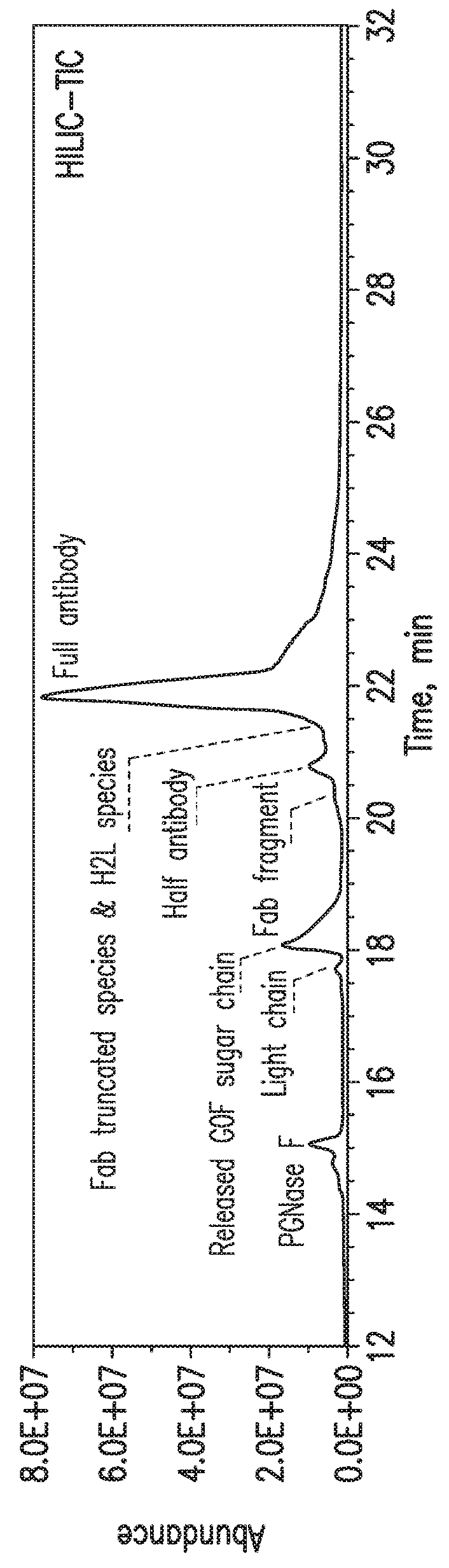
HILIC Separation of mAb-1 Drug Substance Sample
Materials
[0063]For this study, a recombinant IgG1 mAb (mAb-1) made by Regeneron was used. Peptide-N-Glycosidase F (PNGase F, #P0704L) was purchased from New England Biolabs, 1 M Tris-hydrochloride pH 7.5 solution (#15567-027) was purchased from Invitrogen, dithiothreitol (DTT, #20291) was purchased from Thermo Fisher Scientific, and L-cysteine (#168149-25G) was purchased from Sigma-Aldrich. Acetonitrile (LC-MS grade, #A955-4) and trifluoroacetic acid (TFA, #PI28904) were purchased from Fisher Scientific. Milli-Q water was provided in-house.
Methods
[0064]Deglycosylation of mAb-1 and Limited Reduction by DTT and L-Cysteine
[0065]The mAb-1 sample was diluted to a final concentration of 5 μg / μL using 100 mM Tri-HCl (pH 7.5). PNGase F was added at an enzyme to substrate ratio of 1 unit / 10 μg protein. The deglycosylation reaction was conducted at 37° C. for 3 hours. To initiate the limited reduction by DTT, a 20 μg aliquot of the deglycosylated...
example 2
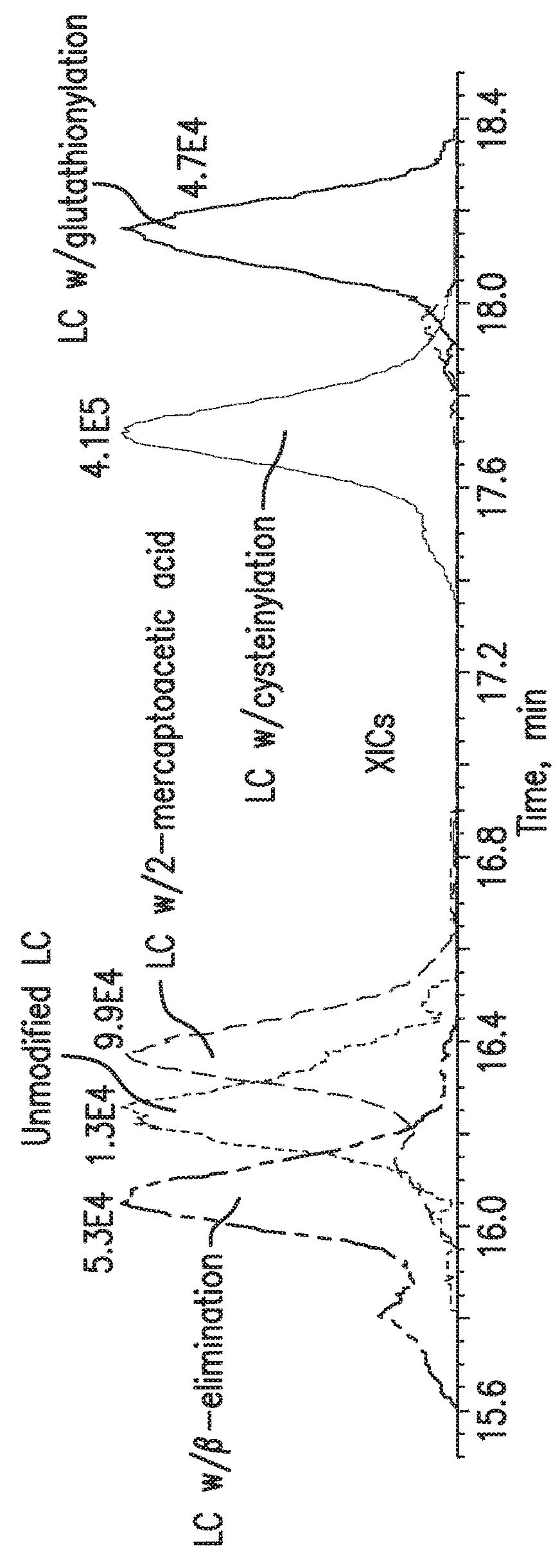
Free Light Chain
Results
[0069]Multiple light chain-related impurities were detected in HILIC-MS analysis of the mAb-1 sample (FIGS. 2A-2E). The extracted ion chromatograms (XICs) of each species suggested that they also exhibited different retention times during HILIC separation (FIG. 2A). Interestingly, the cysteinylated light chain (+˜119 Da) was identified as the major form of all light chain species present in mAb-1 sample and it is the only species visible by UV (FIG. 2D). The cysteinylation may occur as a result of the thiol-disulfide exchange reaction between the inter-heavy and light chain disulfide bond and a free cysteine molecule, which can be found in cytoplasm. In addition, glutathionylated light chain (+˜306 Da) was also identified, with a retention time slightly later than the cysteinylated light chain (FIG. 2E). Similar to the cysteinylation process, free glutathione (GSH) molecule, which can also be found in cytoplasm, should be responsible for this modification. Ano...
example 3
Fab Fragment
Results
[0070]It is well known that the upper hinge region of heavy chains in an IgG1 molecule is susceptible to hydrolysis leading to the formation of two complementary LMW species, a Fab fragment and a Fab-truncated species (Cordoba A J, Shyong B J, Breen D, Harris R J. Journal of chromatography B: Analytical technologies in the biomedical and life sciences, 818:115-21 (2005). The HILIC-MS method detected both species in the mAb-1 sample. Four major Fab fragments with different masses were identified (FIGS. 3A-3D) and the truncation sites were located by comparing the measured masses with the predicted masses, based on the cDNA-derived amino acid sequence. The amino acid pattern indicated in the deconvoluted mass spectrum matched with the heavy chain hinge region sequence (Cys-Asp-Lys-Thr-His-Thr-Cys). It is worth noting that any Fab fragment should have been readily removed during the purification process due to the lack of the Protein A binding site. Therefore, the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap