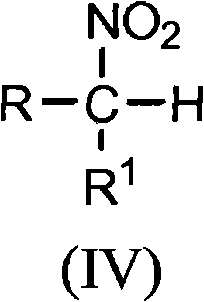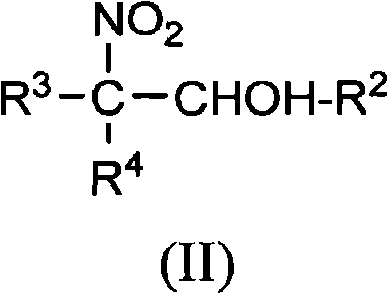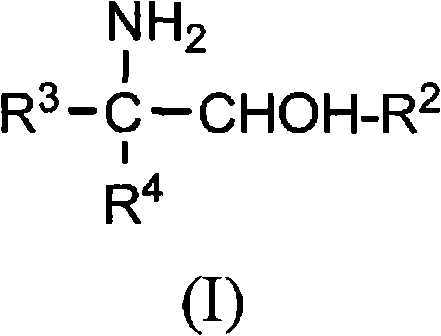Process for making aminoalcohol compounds
A compound, amino alcohol technology, applied in the field of manufacturing amino alcohol compounds, can solve problems such as by-products affecting the color and odor of target materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0041] Examples 1-8: 2-Amino-2-ethyl-1,3-propanediol
[0042] Examples 1-8 relate to 2-amino-2-ethyl-1,3-propanediol (AEPD), which can be prepared from 1-nitropropane and formaldehyde.
[0043] The analysis (characterization) methods used in the examples are as follows.
[0044] GC analysis. The effect of process variations on GC area % was monitored using an HP 5890 Series II Gas Chromatograph with a J&W DB-5 column of 30 m*0.25 mm*1.0 μm. Set the FID detector at 250°C and the injector at 180°C. The column temperature program is: 60°C for 4 minutes, ramp up to 220°C at a constant rate of 30°C / min, hold for 7 minutes, ramp up to 280°C at a constant rate of 20°C / min, and hold for 2 minutes. The injection volume was 1 μL, the split ratio was 100:1, and helium was used as the carrier gas.
[0045] HPLC analysis. The concentrations of the unwanted by-products of the condensation reaction, 2-nitrobutanol (2-NB) and 2-nitro-2-ethyl-1,3-propanediol (NEPD), were determined by HP...
Embodiment 1
[0058] Embodiment 1, this embodiment (control) uses 900.3g NEPD solution. No propylamine was added to the autoclave heel. 1169.3 g of autoclave filtrate was recovered.
Embodiment 2
[0059] Embodiment 2, this embodiment uses 900.1g NEPD solution. To the autoclave residue was added 5 mol.% propylamine (14.1 g). 1138.3 g of autoclave filtrate was recovered.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com