Method for preparing Nintedanib ethylsulfonate
A technology of nintedanib ethanesulfonate and acetyl group, which is applied in the field of preparation of nintedanib ethanesulfonate, can solve problems such as shortage, and achieve the effects of low impurity level, simple preparation process and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
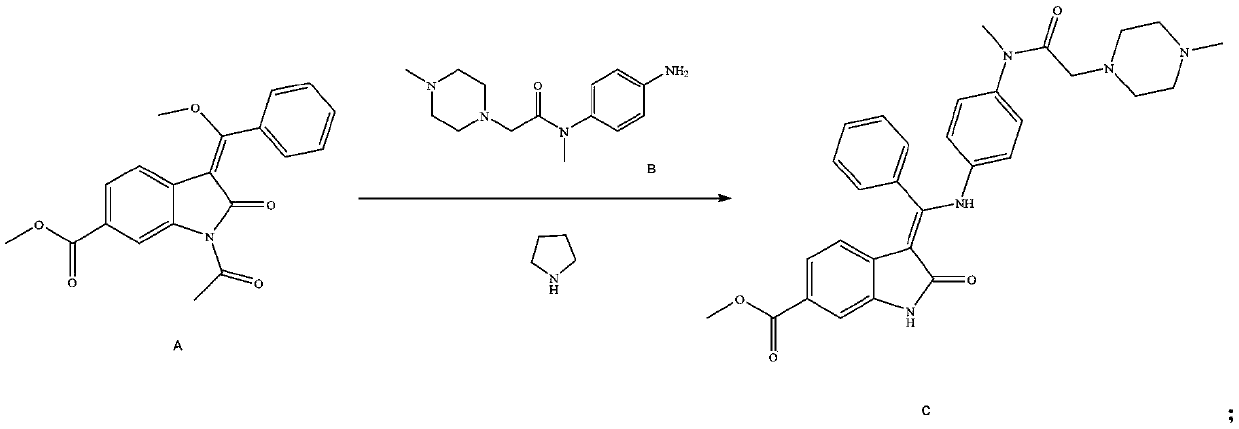
[0050] Add 900ml methanol, 90.0g 1-acetyl-3-[methoxy(phenyl)methylene]-2-oxoindoline-6-formic acid methyl ester (compound A) and 2L glass reaction flask 80.6g compound N-(4-aminophenyl)-N,4-dimethyl-1-piperazineacetamide (compound B), heated to 45-50°C, reacted for 2h, then added 18.2g pyrrolidine, Stir the reaction at 45-50°C for 1h, cool down to 25-30°C, stir and crystallize for 3h, filter, stir wash with a mixed solvent of dichloromethane:methanol with a volume ratio of 10:1, and dry to obtain (3Z)-3-{[ (4-{Methyl-[(4-methylpiperazin-1-yl)acetyl]amino}phenyl}amino}-(phenyl)methylene}-2-oxo-2,3-di Methyl indoline-6-carboxylate (compound C) 126.3 g, yield 91.37%, purity 99.89%.
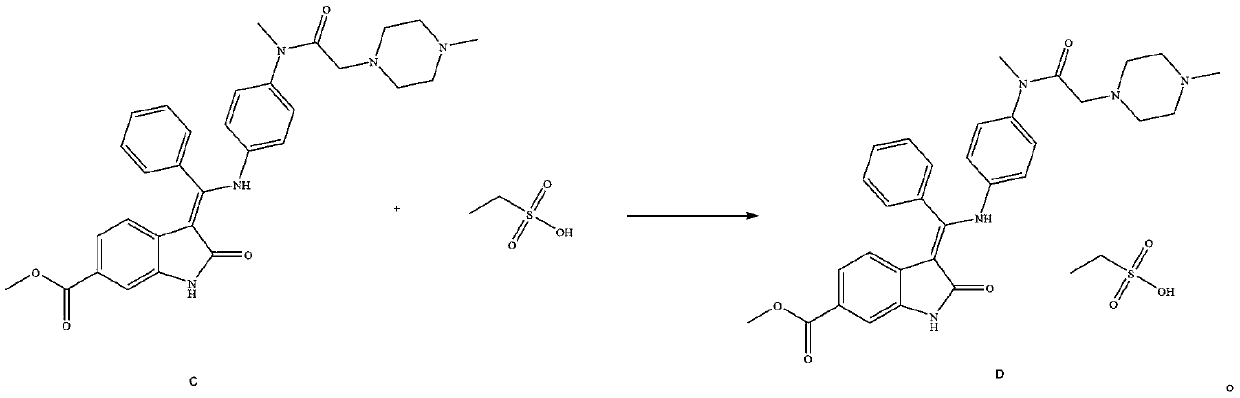
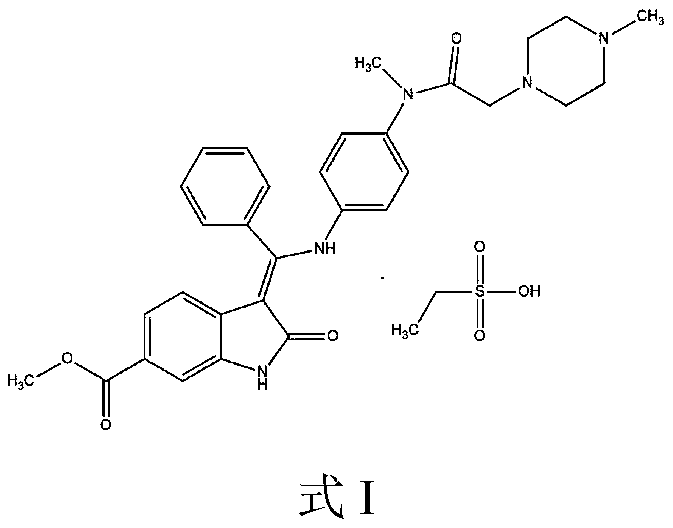
[0051] Add 1.2L of anhydrous methanol to a 2L glass bottle, add 100.0g of (3Z)-3-{[(4-{methyl-[(4-methylpiperazin-1-yl)acetyl] prepared in Example 1 Amino}phenyl}amino}-(phenyl)methylene}-2-oxo-2,3-dihydroindoline-6-carboxylic acid methyl ester (compound C), warming up to 50-55°C, drop 70% ethanes...
Embodiment 2
[0054] Using 900mL N,N-dimethylformamide instead of methanol as the reaction solvent in step 1, the other operations were the same as in Example 1, step 1 obtained compound C124.3g, yield 89.92%, purity 99.85%, step 2 obtained compound D finished product 107.3g, yield 89.11%, purity 99.93%. The total yield of the two-step reaction was 80.1%.
Embodiment 3
[0056] The cooling and crystallization temperature in Step 2 was controlled at 30-35°C, and other operations were the same as in Example 1. In Step 2, 106.2 g of the finished compound D was obtained, with a yield of 88.20% and a purity of 99.95%. The total yield of the two-step reaction was 80.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com